This commentary—authored by Anne Abbott, PhD, MBBS, Lawrence Schott, MD, Lan Gao, MMed, PhD, Hrvoje Budincevic, MD, PhD, Rishad Faruqi, MD, Tatjana Rundek, MD, PhD, Jean-Baptiste Ricco, MD, PhD, Saeid Shahidi, MD, and Gert J. de Borst, MD, PhD—consists of a multinational, multispecialty, multidisciplinary updated evidence review and policy advice, which was published on the U.S. Centers for Medicare & Medicaid Services (CMS) website on Aug. 8, 2023. This review and policy advice was prompted by CMS’ proposed decision to fund “free-for-all” carotid artery “stenting” procedures in any American aged at least 65 years, on certain disability benefits and/or with end-stage renal failure who is determined as having at least “50” or “70%” carotid stenosis (despite no accurate and reproducible method of classifying degree of carotid stenosis). This manuscript is based on a longer group submission posted on the CMS website in February 2023.
This commentary—authored by Anne Abbott, PhD, MBBS, Lawrence Schott, MD, Lan Gao, MMed, PhD, Hrvoje Budincevic, MD, PhD, Rishad Faruqi, MD, Tatjana Rundek, MD, PhD, Jean-Baptiste Ricco, MD, PhD, Saeid Shahidi, MD, and Gert J. de Borst, MD, PhD—consists of a multinational, multispecialty, multidisciplinary updated evidence review and policy advice, which was published on the U.S. Centers for Medicare & Medicaid Services (CMS) website on Aug. 8, 2023. This review and policy advice was prompted by CMS’ proposed decision to fund “free-for-all” carotid artery “stenting” procedures in any American aged at least 65 years, on certain disability benefits and/or with end-stage renal failure who is determined as having at least “50” or “70%” carotid stenosis (despite no accurate and reproducible method of classifying degree of carotid stenosis). This manuscript is based on a longer group submission posted on the CMS website in February 2023.
We classify the types of misinformation being used to errantly push U.S. Medicare to expand reimbursement indications for carotid artery stenting (CAS) and a new hybrid procedure known as transcarotid arterial revascularization (TCAR). This is already an environment with too many unnecessary carotid procedures done in the name of stroke risk reduction. Founding members of the Faculty Advocating Collaborative and Thoughtful Carotid Artery Treatments (FACTCATS) explained in 2012 why reimbursement indications for CAS should not be expanded.1,2 The fundamental reasons were higher risks with CAS (stroke, death and myocardial infarction) compared to carotid endarterectomy (CEA) and declining carotid procedural indications due to greatly improved non-invasive stroke risk reduction methods. The situation has not changed, except that the excessive hazards with stenting are clearer, non-invasive medical intervention for stroke risk reduction has improved further and the safety and efficacy of TCAR remain unproven. Once again, we advise that expanding reimbursement indications for carotid procedures “would have serious negative health and economic repercussions for the USA and any other country that may follow such inappropriate action.”1
Introduction
Since 2005, CMS has limited carotid stenting (CAS) reimbursement indications to individuals considered at high-risk from carotid surgery (carotid endarterectomy, CEA) who are symptomatic with >70% carotid stenosis and to other high-CEA-risk patients if used in appropriate studies (those with 50-70% stenosis if symptomatic or >80% stenosis if asymptomatic).3 High-CEA-risk is defined by CMS as having major comorbidities and/or anatomic risk factors (including current life-threatening cardiac conditions).3 The definition is open to interpretation. CMS also created minimum standards upon which CAS coverage (reimbursement) is dependent.3
As per the January 2023 online CMS announcement,4 a “multispecialty alliance” (the “Alliance”) asked that CMS:
- Expand CAS reimbursement indications to all individuals with >70% asymptomatic carotid stenosis and any symptomatic person with >50% stenosis (including those not considered high-CEA-risk)
- Eliminate the minimal standards for facility requirements
- Leave coverage for any CAS procedure (including TCAR) not described by the CMS National Coverage Determination (NCD) to the discretion of the local Medicare Administrative Contractors
Founding members of FACTCATS published evidence reviews and advice to U.S. Medicare in 2012 and 2013 outlining why reimbursement indications for CAS should not be expanded.1,2 The main reasoning then was the higher risk of stroke and death with CAS compared to CEA, as well as declining carotid procedural indications due to greatly improved non-invasive stroke risk reduction methods (risk factor identification and amelioration using lifestyle interventions and medication). The situation has not changed, except that the excessive hazards with CAS are clearer, non-invasive medical intervention for stroke risk reduction has improved further and the safety and efficacy of TCAR remain unproven.5–10
CMS reimbursement indications were not expanded following the 2012 Medicare Evidence Development & Coverage Advisory Committee (MEDCAC) meeting.5 The results have been greatly limited access to procedural “carotid revascularization” by non-surgically trained practitioners, the saving of up to several million older Americans from unnecessary, excessively dangerous carotid procedures, the saving of up to at least 60 billion American healthcare dollars and an excellent global health policy example.5,11 This analysis is a cut-down version of our updated evidence review and policy advice for CMS that was publicly posted Feb. 10, 2023, with 71 co-signatories.
The Alliance used an online letter to CMS and a 2022 review article as the case for ‘new evidence’ supporting expansion of reimbursement indications for CAS, TCAR and any other procedure they may consider under the umbrella of CAS in the future.4,12,13 However, the case for expansion consists of misinformation favoring procedural over-use and lacks a cost-effectiveness analysis. We have classified the most important forms of misinformation encouraging procedural overuse into four types: factual errors (untrue statements), fact distortion (a fact is conveyed then misconstrued), omitted information (omission of information relevant to clinical decision making) and speculation. We would like to emphasize that we are not targeting individuals, rather we are addressing misinformation. Further, this misinformation is common in the medical literature.
Factual errors
- CAS is not equivalent, or noninferior, to CEA as claimed12,13
CAS is more dangerous for patients than CEA. In every adequately powered randomized trial comparison, CAS (when used for primary or secondary stroke risk reduction) was associated with approximately 1.5–2 times as many peri-procedural strokes or deaths as CEA.5 Several individual randomized trials of symptomatic patients showed a statistically significant higher periprocedural rate of stroke or death from CAS compared to CEA (including the International Carotid Stenting Study [ICSS], the Carotid Revascularization Endarterectomy vs. Stenting Trial [CREST-1], and the Endarterectomy Versus Angioplasty in Patients with Symptomatic Severe Carotid Stenosis [EVA-3-S] trial).5 Registries and administrative databases have also consistently shown higher periprocedural rates of stroke or death with CAS compared to CEA.2,5,14
By contrast, no individual randomized trial of CAS versus CEA in patients with asymptomatic (or recently asymptomatic) carotid stenosis has been sufficiently powered for this comparison because of low event rates and, hence, the requirement for larger study samples.5,15,16 Underpowered trials involving asymptomatic patients include the 2nd Asymptomatic Carotid Surgery Trial (ACST-2), published in 2021.17,18 However, all individual randomized trials of asymptomatic, or recently asymptomatic patients, with more than 200 subjects have shown trends towards more periprocedural stroke or death with CAS than CEA. For example, in ACST-2, the odds ratio (OR) for 30-day peri-procedural death or stroke with CAS versus CEA was 1.35 (95% confidence interval [CI] 0.91–2.03). The comparison reached statistical significance in a 2019 meta-analysis of randomized trials (eight trials and 3,467 patients: OR 1.64, 95% CI 1.02–2.64)15 and borderline significance in a 2020 meta-analysis of randomized trials (seven trials and 3,378 patients: OR 1.72, 95% CI 1.00–2.97).16
In randomized trials, myocardial infarction was generally less common with CAS than CEA. However, where published, in randomized trials of CAS versus CEA involving symptomatic and/or asymptomatic patients, peri-procedural stroke (mostly caused by CAS) was overall 4.6 times more common than clinically defined myocardial infarction.19 Further, periprocedural death was 34% higher with CAS and periprocedural stroke, death, and clinically defined myocardial infarction were 1.6 times more common after CAS than CEA.5,19 The difference in this latter, triple-composite outcome measure reached statistical significance in meta-analyses involving symptomatic patients.5,15,16
There are still insufficient randomized trial data to adequately power this triple-composite comparison for asymptomatic patients.5,15,16 However, randomized trial results indicate that it is highly likely that if CAS is rolled out into routine practice (involving many more asymptomatic patients than in trials with/or without lower procedural standards) it would cause significantly more strokes, deaths and myocardial infarctions than CEA. That would be clinically and economically highly significant.5
Further, in adequately powered randomized trial comparisons, rates of periprocedural stroke or death and later ipsilateral stroke have been higher with CAS compared to CEA for as long as patients have been followed up.5 Rates of new ipsilateral stroke beyond the periprocedural period were generally similar with each procedure. This indicates that patients who have a periprocedural stroke tend to live long-term with their stroke. Meanwhile, those that die in the periprocedural period do not recover. Therefore, compared to CEA, the harm from CAS is durable.5 Differing results have been reported with respect to ‘protection devices’ for lowering the CAS-associated risk of stroke or death.5 Further, severe carotid re-stenosis is more common after CAS than CEA and CAS tends to cost more.20,21 Complications (apart from stroke and death) that are more likely with, or particular to, CAS compared to CEA include hemodynamic instability (severe hypotension or bradycardia, including the need for a permanent pacemaker) and retroperitoneal haemorrhage.5
- Age is not the only risk factor for harm from CAS as claimed12,13
In randomized trials, CAS has not been shown to be more beneficial than CEA or non-invasive medical intervention alone in any subgroup of patients. Particularly vulnerable to stroke from CAS compared to CEA are:
- The most senior patients (aged >70 years). Randomized trial comparisons in younger patients have been underpowered, probably because of lower event rates and less representation5
- Those who are most recently symptomatic (especially within the previous 7–14 days, which is when best practice CEA is most likely to be beneficial)5,22
- Women. However, men are also at higher risk of stroke or death from CAS compared to CEA5
- Those with certain carotid anatomical features, such as longer, angulated, or tandem lesions5
- Those who have CAS in low volume centers or outside trials5
- Regression of carotid plaque has been seen from non-invasive medical intervention alone, in contrast to what was claimed12,13
Studies have shown that statin and ezetimibe therapy can cause carotid plaque regression and content stabilization.23-28 Moreover, statins, ezetimibe and PCSK9 inhibitors reduce the risk of stroke and other arterial disease morbidity and mortality whether or not individuals undergo a carotid artery procedure.29–31
Fact distortion
- Inappropriate dismissal of ‘minor’ or ‘nondisabling’ strokes in attempt to justify CAS12,13
The most severe or fatal periprocedural strokes are usually defined as being associated with a modified Rankin score of >3.5 Fortunately, severe strokes are less common than milder strokes. A well-recognized method of falsely claiming equivalence between CAS and CEA is to ignore milder strokes.19 However, this distorts the facts in two ways. Firstly, so-called ‘minor’ strokes may impose significant disability and reduced quality of life and should not be discounted.32,33 Secondly, past randomized trials of CAS versus CEA were underpowered to compare the peri-procedural rate of the most severe strokes. Therefore, it is incorrect to claim that CAS and CEA are similar with respect to causing severe strokes.5
- Inappropriate use of periprocedural myocardial infarction to justify CAS12,13
As explained above, in all adequately powered randomized trial comparisons, CAS was associated with significantly more periprocedural stroke, death and clinically defined myocardial infarction than CEA.15,16,19 However, because myocardial infarction was often more frequent with CAS than CEA in randomized trials, including it in a primary outcome measure with stroke and death means the adverse outcomes are more evenly distributed between CEA and CAS. Therefore, larger sample sizes are required to show statistically significant procedural differences. As explained above, this has been achieved in meta-analyses of randomized symptomatic patients.15,16 Underpowered triple-composite outcome comparisons camouflage the statistically significant higher periprocedural rate of stroke or death with CAS compared to CEA. “No statistically significant difference” is often due to underpowering, and this is demonstrated with meta-analyses involving larger subject numbers.5,15,16,19
- Inappropriately using the SAPPHIRE trial to justify CAS in high-CEA-risk patients12,13
The Stenting and Angioplasty with Protection in Patients at High Risk for Endarterectomy (SAPPHIRE) trial was inappropriate to determine a role for carotid artery procedures in individuals considered at high-CEA-risk because it lacked a non-invasive-treatment-only option.34 Performing randomized trials of only procedures and concluding that a procedure is best, or indicated, constitutes procedural bias.19,35 Outcomes with non-invasive treatment alone have not been measured in these patients. Further, high-surgical-risk medical comorbidities (such as unstable angina, congestive cardiac failure and advanced age) identify individuals with high rates of procedural complications and not likely to live long enough to benefit from a carotid artery procedure.36-40 Interestingly, patients deemed high-CEA-risk using CMS criteria do not appear better off with CAS compared to CEA.41
The SAPPHIRE trial had other major limitations for applicability in clinical practice. It included myocardial infarction in the primary outcome measure (the pitfalls of this are described above). Further, myocardial infarction was unusually defined as a “creatinine kinase level >2 times the upper limit of normal with a positive myocardial band (MB) fraction.”34 However, elevated cardiac enzymes are not necessarily indicative of significant heart damage, are not rare after non-cardiac surgery, and are perhaps more common after CEA than CAS.42-44 Further, the SAPPHIRE sample size was very small (334, 70% asymptomatic) with only 17 total 30-day periprocedural strokes or deaths (OR for CAS versus CEA: 0.9, 95% CI 0.3–2.3) and 55 total strokes or deaths by one year (OR for CAS versus CEA: 0.6, 95% CI 0.3–1.1). The trial was underpowered, particularly with respect to analysis of asymptomatic and symptomatic patients separately. As far as we know, the SAPPHIRE trial is the only randomized procedural trial of high-CEA-risk patients.12,13 SAPPHIRE’s limitations, and unknown outcomes with current best practice non-invasive treatment alone, underscore the lack of evidence supporting a procedural approach in “high-surgical-risk” individuals and an outstanding over-reliance on SAPPHIRE in health policy and guidelines.45
- Inappropriately using outdated and overreaching guidelines to justify CAS12,13
It is inappropriate to cite outdated procedural standards (such as a 30-day periprocedural stroke or death rate <2–3% for asymptomatic carotid stenosis patients or <6% for stroke or TIA patients with carotid stenosis) to encourage ongoing routine use of carotid artery procedures, let alone reimbursement expansion. The randomized trials upon which such guidelines and standards are based are long outdated due to ongoing advances in non-invasive stroke prevention.5,45 Further, prevailing guidelines encourage carotid artery procedures for many more patient subgroups than have ever been shown to benefit (see below).5,45
- Patient choice in treatment decisions12,13
The Alliance repeatedly referred to patient choice, or preference, as a reason to expand reimbursement indications. Patient preference, and informed consent, are prerequisites for any medical intervention.46 This is usually the last “bastion” in protecting patients.46 However, patient preference strongly depends on the way information is presented (or omitted). This has already been demonstrated with decision-making in asymptomatic carotid stenosis patients.47 This demonstrates the importance of how physicians talk to patients. Meanwhile, “shared decision-making” could be a way of making patients take responsibility for treatment that, rather than help, is more likely to harm them.
Omitted information
- Implications of improved non-invasive risk reduction12,13
The efficacy of any carotid procedure in stroke risk reduction can only be established using sufficiently powered comparisons against current best practice non-invasive medical intervention alone (healthy lifestyle habits and appropriate medication). This point was consistently missed by the Alliance. Mention was made in the 2022 review of very low (approximately 1%) annual stroke rates in asymptomatic carotid stenosis patients leading to new trials (CREST-2 and the 2nd European Carotid Surgical Trial [ECST-2]) comparing procedures to non-invasive treatment alone.13 In the Alliance letter of request, “expanded” non-invasive medical intervention was deemed “experimental,” instead of the gold standard against which all procedures should be compared.5,12
The ‘Alliance’ advocating for expansion of CAS reimbursement neglected to explain an evidence base showing that stroke rates have dropped by >50-65% with non-invasive care alone in asymptomatic and symptomatic individuals with carotid stenosis, and other cerebrovascular disease populations, since all past randomized trials of CEA versus non-invasive treatment alone.5,7,8,48–53 The Alliance did not mention that these outstanding achievements with non-invasive care mean that there is no current evidence of patient benefit from any carotid procedure. However, there are ongoing reported rates of periprocedural stroke or death of >1–6%.14 Even if procedural stroke and death rates of consistently zero were possible, this is insufficient to justify carotid procedures in the absence of a clinically significant benefit compared to current best-practice non-invasive care alone.5,54
- Degree of 50–99% asymptomatic carotid stenosis does not justify a procedure12,13
The Alliance cited a recent Oxford Vascular Study publication describing how patients with 80–99%, compared to 50–79%, asymptomatic carotid stenosis had a higher ipsilateral stroke rate.12,13,55 However, they did not explain that the highest average annual ipsilateral stroke rate seen in that study approximated only 3%, not high enough to ensure a procedural benefit if Asymptomatic Carotid Atherosclerosis Study (ACAS) results are used as an indicator.51,54,56 Furthermore, they neglected to explain that the non-invasive stroke prevention treatment used in that study (and particularly in the associated meta-analysis studies) was suboptimal. Therefore, it is highly likely that the 3% average annual ipsilateral stroke rate is higher than that achievable with current best practice non-invasive care alone.54
- TCAR has not been shown to be as safe as CEA or more effective than non-invasive care12,13
Even with large numbers of registry patients, there is no clear evidence that TCAR is as safe as CEA in terms of the risk of stroke or death, at least in standard-surgical-risk patients.32,57–61 TCAR (like CEA) appears to be safer than CAS in the Vascular Quality Initiative (VQI) registry 57–59 However, definitive interpretation of TCAR studies is impossible because of underpowering, lack of randomization, mismatch in patient comorbidity loads, combined patient symptomatic status, predominance of asymptomatic patients, retrospective interrogation and lack of comparison with current best practice non-invasive care.9,10,61,62 CEA remains the safest carotid revascularization procedure and should still be considered in selected symptomatic patients.5,10 However, patients and their carers need to understand that the evidence for an overall CEA benefit compared to non-invasive care alone was collected decades ago and needs re-evaluation.5,63
- Differentiating ‘asymptomatic’ and ‘symptomatic’ and those shown to benefit12,13
The Alliance request expansion of reimbursement in “selected candidates.” However, no details are provided about how to select patients. Definitions are not given for asymptomatic or symptomatic patients or for categorizing degree of carotid stenosis.12,13Further, the Alliance omitted to explain the patient subgroups ever shown to have an overall benefit from a procedure (only CEA) compared to non-invasive treatment alone in randomized trials.48–51,64 The overall CEA benefit was small (approximately 1%/year for asymptomatic/recently asymptomatic carotid stenosis patients and up to approximately 3.2%/year for symptomatic patients with ipsilateral carotid stenosis).5 Further, patients had to satisfy all trial selection criteria, have a life expectancy of at least 3–5 years and fit into one of the four following groups:5
- Men aged <75–80 years with 60–99% asymptomatic (or recently asymptomatic) carotid stenosis (defined using conventional intra-arterial angiography or ultrasound and North American Symptomatic Carotid Endarterectomy Trial [NASCET] criteria)51,64
- Symptomatic Women with 70–99% stenosis (defined using conventional intra-arterial angiography and NASCET criteria and without near occlusion) having CEA within 2–3 weeks of their last same-sided non-severe stroke or TIA)65
- Symptomatic Men with 50–69% stenosis (defined using conventional intra-arterial angiography and NASCET criteria) having CEA within 2–3 weeks of their last same-sided non-severe stroke or TIA65
- Symptomatic Men with 70–99% stenosis (defined using conventional intra-arterial angiography and NASCET criteria and without near occlusion) having CEA within 3 months of their last same-sided non-severe stroke or TIA. However, the CEA benefit fell rapidly over this time and was highest within the first 2–3 weeks65
There has never been evidence of procedural efficacy for any other subpopulation of carotid stenosis patients. Further, evidence for the groups defined above is outdated. A “lean to” approach with respect to procedural reimbursement (or “access”) in other subpopulations is inappropriate, given the large numbers of additional implicated patients, and especially when there is evidence of net harm.5 Prevailing policy should be reevaluated with appreciation of the health and economic importance of appropriate “exnovation” and independent monitoring of outcomes with all treatment modalities (procedural and nonprocedural).5,66 Further, it needs to be recognized that a randomized trial is not always needed to answer clinical question. For example, if ipsilateral stroke rates associated with carotid arterial disease are clearly sufficiently close to zero with non-invasive care alone, then randomized procedural trials are not required.5
- Categorizing degree of stenosis (and risk) has changed, and is fraught with error12,13
The dominant method of measuring carotid stenosis in previous randomized trials was catheter-based digital subtraction angiography and the NASCET criteria for stenosis.67 Ultrasound was used to some extent in ACAS and was used in ACST-1 (the 1st Asymptomatic Carotid Surgery Trial).51,64 However, most arterial imaging is now non-invasive. Furthermore, there is inherent error with each imaging modality and correlation is far from perfect.5,68,69 Concerningly, angiography using magnetic resonance imaging and computed tomography, and “real world” operators, tend to overestimate carotid stenosis severity, encouraging procedural overuse.70–72 Changes in imaging methodology for categorizing degree of carotid stenosis and measuring risk, along with major advances in noninvasive care and the lack of enforcement of procedural standards outside trials, are major reasons why the original randomized trials of CEA versus non-invasive intervention alone are outdated and/or non-applicable, and the Alliance requests are inappropriate.5,73–75
Speculation
It is speculation that outcomes with CAS may improve.12,13 This speculation is not relevant to funding decisions currently under discussion. In fact, the evidence base indicates that CAS (at least by the transaortic route) should be avoided.
Conclusions
In summary, rather than expansion, the evidence base clearly demonstrates that it is time for exnovation when it comes to reimbursement indications for carotid artery procedures.66 There are many reasons, and any reason alone could lead to net patient harm:5
- Prevailing guidelines advocate for carotid procedures on many more subgroups than have ever been shown to benefit.
- CAS is more dangerous than CEA, and TCAR has not been shown to be as safe as CEA.
- Procedural complication rates, when measured, are usually higher in practice than in trials.
- There is no current randomized trial evidence of carotid procedural benefit for any patient subgroup compared to current best practice non-invasive care alone. Further, all patients should receive current best practice non-invasive care. Plus, randomized trials are not required to answer every clinical question. For example, if ipsilateral stroke rates are clearly sufficiently close to zero with non-invasive care alone, then randomized procedural trials in such patients are unnecessary and may be unethical.
- Methods of categorizing carotid stenosis have changed and lack reproducibility.
- Routine procedural reimbursement blocks new, critically needed research (such as CREST-2 and the Stent-Protected Angioplasty in Asymptomatic Carotid Artery Stenosis vs. Endarterectomy [SPACE-2] trial) to assess carotid procedural efficacy against non-invasive care alone.5,76
- Healthcare system sustainability in the U.S. (and elsewhere) is threatened by rising costs, including from poor quality care.77,78
CMS, and other payers of health services, are key in protecting patients and healthcare resources. This is particularly pertinent in an environment already compromised by misinformation and self-serving practices.35,79,80 Overuse of carotid artery procedures is a global problem driven by entrenched misdirected incentives.5,81,82 It can be overcome by redirecting wasted resources to effective interventions, new research, and establishing outcomes-based, rather than activity-based, medicine.5,83 Patient welfare focused medicine requires putting aside pressure from those likely to gain directly or indirectly, in terms of funds and/or popularity, and objectively sorting the scientific facts. Further, it means insisting on independently maintained clinical standards which ensure healthcare excellence, free from conflict of interest, and which protect patients from harmful or unproven treatment.
References
- Abbott AL, Adelman MA, Alexandrov AV, Barnett HJ, Beard J, Bell P, Bjorck M, Blacker D, Buckley CJ, Cambria RP, et al. Why the United States Center for Medicare and Medicaid Services (CMS) should not extend reimbursement indications for carotid artery angioplasty/stenting. Eur J Vasc Endovasc Surg. 2012;43:247-251.
- Abbott AL, Adelman MA, Alexandrov AV, Barber PA, Barnett HJ, Beard J, Bell P, Bjorck M, Blacker D, Bonati LH, et al. Why calls for more routine carotid stenting are currently inappropriate: an international, multispecialty, expert review and position statement. Stroke. 2013;44:1186-1190.
- US Centers for Medicare & Medicaid Services (CMS). National Coverage Determination [NCD] for Percutaneous Transluminal Angioplasty [PTA] 20.7. Available at: https://www.cms.gov/medicare-coverage-database/view/ncd.aspx?ncdid=201&ncdver=10.
- US Centers for Medicare & Medicaid Services (CMS). Percutaneous trans-luminal angioplasty (PTA) of the carotid artery concurrent with stenting: National coverage analysis. CAG–00085R8. Available at: https://www.cms.gov/medicare-coverage-database/view/ncacal-tracking-sheet.aspx?ncaid=311. 12th January, 2023.
- Abbott A. Extra-Cranial Carotid Artery Stenosis: An Objective Analysis of the Available Evidence. Front Neurol 2022 Jun 21;13:739999
- Yang C, Bogiatzi C, Spence JD. Risk of Stroke at the Time of Carotid Occlusion. JAMA Neurology. 2015;72:1261-1267.
- Shahidi S, Owen-Falkenberg A, Gottschalksen B, Ellemann K. Risk of early recurrent stroke in symptomatic carotid stenosis after best medical therapy and before endarterectomy. Int J Stroke. 2016;11:41-51.
- Fisch U, von Felten S, Wiencierz A, Jansen O, Howard G, Hendrikse J, Halliday A, Fraedrich G, Eckstein HH, Calvet D, et al. Risk of Stroke before Revascularisation in Patients with Symptomatic Carotid Stenosis: A Pooled Analysis of Randomised Controlled Trials. Eur J Vasc Endovasc Surg. 2021;61:881-887.
- de Borst GJ. Transcarotid Artery Stenting: Hype or Hope? Stroke. 2022;53:108-110. doi: 10.1161/STROKEAHA.121.036464
- de Borst GJ. Transcarotid arterial revascularisation. BJS. 2023;110:127–128.
- Abbott AAL. Healthy lifestyle habits and appropriate medication: Modern breakthroughs in reducing risk of stroke and other arterial disease complications. Veithsymposium Bulletin. June, 2022: https://www.veithsymposium.org/pdf/articles/vei/61.pdf.
- Brott T, Clair DG, Gray W, Heck D, Jovin T, Lyden S, Metzger C, Rosenfield K, Roubin G, Sachar R, et al. Formal Request for Reconsideration of National Coverage Determination (NCD) 20.7, 2022: https://www.cms.gov/Medicare/Coverage/DeterminationProcess/downloads/id311.pdf..
- White CJ, Brott TG, Gray WA, Heck D, Jovin T, Lyden SP, Metzger DC, Rosenfield K, Roubin G, Sachar R, et al. Carotid Artery Stenting: JACC State-of-the-Art Review. Journal of the American College of Cardiology. 2022;80:155-170.
- Paraskevas KI, Kalmykov EL, Naylor AR. Stroke/Death Rates Following Carotid Artery Stenting and Carotid Endarterectomy in Contemporary Administrative Dataset Registries: A Systematic Review. Eur J Vasc Endovasc Surg. 2016;51:3-12.
- Batchelder AJ, Saratzis A, Ross Naylor A. Overview of Primary and Secondary Analyses From 20 Randomised Controlled Trials Comparing Carotid Artery Stenting With Carotid Endarterectomy. Eur J Vasc Endovasc Surg. 2019;58:479-493.
- Muller MD, Lyrer P, Brown MM, Bonati LH. Carotid artery stenting versus endarterectomy for treatment of carotid artery stenosis. Cochrane Database Syst Rev. 2020;2:CD000515.
- Abbott AL, Wijeratne T, Zeebregts CJ, Ricco JB, Svetlikov A. Is stenting equivalent to endarterectomy for asymptomatic carotid stenosis? Lancet. 2022;399:1115-1116.
- Halliday A, Bulbulia R, Bonati LH, Chester J, Cradduck-Bamford A, Peto R, Pan H, for the ACST-Collaborative Group. Second asymptomatic carotid surgery trial (ACST-2): a randomised comparison of carotid artery stenting versus carotid endarterectomy. Lancet. 2021;398:1065-1073.
- Abbott AL, Brunser AM, Giannoukas A, Harbaugh RE, Kleinig T, Lattanzi S, Poppert H, Rundek T, Shahidi S, Silvestrini M, et al. Misconceptions regarding the adequacy of best medical intervention alone for asymptomatic carotid stenosis. J Vasc Surg. 2020;71:257-269.
- Eslami MH, McPhee JT, Simons JP, Schanzer A, Messina LM. National trends in utilization and postprocedure outcomes for carotid artery revascularization 2005 to 2007. J Vasc Surg. 2011;53:307-315.
- Cole TS, Mezher AW, Catapano JS, Godzik J, Baranoski JF, Nakaji P, Albuquerque FC, Lawton MT, Little AS, Ducruet AF. Nationwide Trends in Carotid Endarterectomy and Carotid Artery Stenting in the Post-CREST Era. Stroke. 2020;51:579-587.
- Rantner B, Goebel G, Bonati LH, Ringleb PA, Mas JL, Fraedrich G, Carotid Stenting Trialists C. The risk of carotid artery stenting compared with carotid endarterectomy is greatest in patients treated within 7 days of symptoms. J Vasc Surg. 2013;57:619-626 e612; discussion 625-616.
- Ainsworth CD, Blake CC, Tamayo A, Beletsky V, Fenster A, Spence JD. 3D ultrasound measurement of change in carotid plaque volume: a tool for rapid evaluation of new therapies. Stroke. 2005;36:1904-1909.
- Makris GC, Lavida A, Nicolaides AN, Geroulakos G. The effect of statins on carotid plaque morphology: a LDL-associated action or one more pleiotropic effect of statins? Atherosclerosis. 2010;213:8-20.
- Migrino RQ, Bowers M, Harmann L, Prost R, LaDisa JF, Jr. Carotid plaque regression following 6-month statin therapy assessed by 3T cardiovascular magnetic resonance: comparison with ultrasound intima media thickness. J Cardiovasc Magn Reson. 2011;13:37.
- Bogiatzi C, Spence JD. Ezetimibe and regression of carotid atherosclerosis: importance of measuring plaque burden. Stroke. 2012;43:1153-1155.
- Kakkos SK, Nicolaides AN, Charalambous I, Thomas D, Giannopoulos A, Naylor AR, Geroulakos G, Abbott AL, Asymptomatic Carotid S, Risk of Stroke Study G. Predictors and clinical significance of progression or regression of asymptomatic carotid stenosis. J Vasc Surg. 2014;59:956-967 e951.
- Du R, Cai J, Zhao XQ, Wang QJ, Liu DQ, Leng WX, Gao P, Wu HM, Ma L, Ye P. Early decrease in carotid plaque lipid content as assessed by magnetic resonance imaging during treatment of rosuvastatin. BMC Cardiovascular Disorders. 2014;14:83.
- Raman G, Kitsios GD, Moorthy D, Hadar N, Dahabreh IJ, O’Donnell TF, Thaler DE, Feldmann E, Lau J. Management of Asymptomatic Carotid Stenosis: Technology Assessment Report. 2012.
- Paraskevas KI, Mikhailidis DP, Veith FJ. Statins induce regression of carotid artery stenosis: Fact or fiction? Int J Card. 2016;220:680.
- Giugliano RP, Pedersen TR, Saver JL, Sever PS, Keech AC, Bohula EA, Murphy SA, Wasserman SM, Honarpour N, Wang H, et al. Stroke Prevention With the PCSK9 (Proprotein Convertase Subtilisin-Kexin Type 9) Inhibitor Evolocumab Added to Statin in High-Risk Patients With Stable Atherosclerosis. Stroke. 2020;51:1546-1554.
- Abbott A. Words of Caution Regarding Safety Comparisons Between Transcarotid Artery Revascularization, Carotid Endarterectomy, and Carotid Stenting. JAHA. 2022;11:e027402.
- Brott TG, Hobson RW, 2nd, Howard G, Roubin GS, Clark WM, Brooks W, Mackey A, Hill MD, Leimgruber PP, Sheffet AJ, et al. Stenting versus endarterectomy for treatment of carotid-artery stenosis. New Eng J Med. 2010;363:11-23.
- Yadav JS, Wholey MH, Kuntz RE, Fayad P, Katzen BT, Mishkel GJ, Bajwa TK, Whitlow P, Strickman NE, Jaff MR, et al. Protected carotid-artery stenting versus endarterectomy in high-risk patients. New Eng J Med. 2004;351:1493-1501.
- Abbott AL. Bias in the use of randomized trials for carotid stenosis management. Gefasschirurgie. 2015;20:252-257.
- Wallaert JB, De Martino RR, Finlayson SR, Walsh DB, Corriere MA, Stone DH, Cronenwett JL, Goodney PP. Carotid endarterectomy in asymptomatic patients with limited life expectancy. Stroke. 2012;43:1781-1787.
- Conrad MF, Kang J, Mukhopadhyay S, Patel VI, LaMuraglia GM, Cambria RP. A risk prediction model for determining appropriateness of CEA in patients with asymptomatic carotid artery stenosis. Ann Surg. 2013;258:534-538; discussion 538-540.
- Wallaert JB, Cronenwett JL, Bertges DJ, Schanzer A, Nolan BW, De Martino R, Eldrup-Jorgensen J, Goodney PP, Vasc Study Grp New E. Optimal selection of asymptomatic patients for carotid endarterectomy based on predicted 5-year survival. J Vasc Surg. 2013;58:112-118.
- Melin AA, Schmid KK, Lynch TG, Pipinos, II, Kappes S, Longo GM, Gupta PK, Johanning JM. Preoperative frailty Risk Analysis Index to stratify patients undergoing carotid endarterectomy. J Vasc Surg. 2015;61:683-689.
- Pandip V, Lee A, Zeesham M, Goshima K, Tan TW, Jhajj S, Trinidad B, Weinkauf C, Zhou W. Effective frailty syndrome on outcomes of patients with carotid stenosis. J Vasc Surg. 2020;71:1595-1600.
- Schermerhorn ML, Fokkema M, Goodney P, Dillavou ED, Jim J, Kenwood CT, Siami FS, White RA, Committee SVSO. The impact of Centers for Medicare and Medicaid Services high-risk criteria on outcome after carotid endarterectomy and carotid artery stenting in the SVS Vascular Registry. J Vasc Surg. 2013;57:1318-1324.
- Motamed C, Motamed-Kazerounian G, Merle JC, Dumerat M, Yakhou L, Vodinh J, Kouyoumoudjian C, Duvaldestin P, Becquemin JP. Cardiac troponin I assessment and late cardiac complications after carotid stenting or endarterectomy. J Vasc Surg. 2005;41:769-774.
- Blackshear JL, Cutlip DE, Roubin GS, Hill MD, Leimgruber PP, Begg RJ, Cohen DJ, Eidt JF, Narins CR, Prineas RJ, et al. Myocardial infarction after carotid stenting and endarterectomy: results from the carotid revascularization endarterectomy versus stenting trial. Circulation. 2011;123:2571-2578.
- Moghadam-Kia S, Oddis C, Aggarwal R. Approach to asymptomatic creatinine kinase elevation. Cleveland Clinic Journal of Medicine. 2016;83:37-42.
- Abbott AL, Paraskevas KI, Kakkos SK, Golledge J, Eckstein HH, Diaz-Sandoval LJ, Cao L, Fu Q, Wijeratne T, Leung TW, et al. Systematic Review of Guidelines for the Management of Asymptomatic and Symptomatic Carotid Stenosis. Stroke. 2015;46:3288-3301.
- Abbott AL. Management of Patients with Asymptomatic Carotid Stenosis May Need to Be Individualized: A Multidisciplinary Call for Action. Journal of Stroke. 2022;24:160-162. doi: https://doi.org/10.5853/jos.2021.03034
- Silver B, Zaman IF, Ashraf K, Majed Y, Norwood EM, Schuh LA, Smith BJ, Smith RE, Schultz LR. A randomized trial of decision-making in asymptomatic carotid stenosis. Neurology. 2012;78:315-321.
- Hobson RW, 2nd, Weiss DG, Fields WS, Goldstone J, Moore WS, Towne JB, Wright CB. Efficacy of carotid endarterectomy for asymptomatic carotid stenosis. The Veterans Affairs Cooperative Study Group. New Eng J Med. 1993;328:221-227.
- Barnett HJ, Taylor DW, Eliasziw M, Fox AJ, Ferguson GG, Haynes RB, Rankin RN, Clagett GP, Hachinski VC, Sackett DL, et al. Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. New Eng J Med.1998;339:1415-1425.
- European Carotid Surgery Trialists’ Collaborative Group t. Randomised trial of endarterectomy for recently symptomatic carotid stenosis: final results of the MRC European Carotid Surgery Trial (ECST). Lancet. 1998;351:1379-1387.
- Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. Endarterectomy for asymptomatic carotid artery stenosis. Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. JAMA. 1995;273:1421-1428.
- Abbott AL. Symptomatic Patients and Stroke Risk Before Carotid Endarterectomy or Stenting. Eur J Vasc Endovasc Surg. 2021. Nov;62(5):825
- Chang RW, Tucker LY, Rothenberg KA, Lancaster E, Faruqi RM, Kuang HC, Flint AC, Avins AL, Nguyen-Huynh MN. Incidence of Ischemic Stroke in Patients With Asymptomatic Severe Carotid Stenosis Without Surgical Intervention. JAMA. 2022;327:1974-1982.
- Abbott A. Asymptomatic carotid stenosis and stroke risk. Lancet Neurol. 2022;20:698-699.
- Howard DPJ, Gaziano L, Rothwell PM, Oxford Vascular Study. Risk of stroke in relation to degree of asymptomatic carotid stenosis: a population-based cohort study, systematic review, and meta-analysis. Lancet Neurol. 2021;20:193-202.
- Abbott AL. Medical (nonsurgical) intervention alone is now best for prevention of stroke associated with asymptomatic severe carotid stenosis: results of a systematic review and analysis. Stroke. 2009;40:e573-583.
- Columbo JA, Martinez-Camblor P, Stone DH, Goodney PP, O’Malley AJ. Procedural Safety Comparison Between Transcarotid Artery Revascularization, Carotid Endarterectomy, and Carotid Stenting: Perioperative and 1-Year Rates of Stroke or Death. JAHA. 2022;11:e024964.
- Zhang GQ, Bose S, Stonko DP, Abularrage CJ, Zarkowsky DS, Hicks CW. Transcarotid artery revascularization is associated with similar outcomes to carotid endarterectomy regardless of patient risk status. J Vasc Surg. 2022;76:474-481 e473.
- Cui CL, Dakour-Aridi H, Lu JJ, Yei KS, Schermerhorn ML, Malas MB. In-Hospital Outcomes of Urgent, Early, or Late Revascularization for Symptomatic Carotid Artery Stenosis. Stroke. 2022;53:100-107.
- Liang P, Cronenwett JL, Secemsky EA, Eldrup-Jorgensen J, Malas MB, Wang GJ, Nolan BW, Kashyap VS, Motaganahalli RL, Schermerhorn ML. Risk of Stroke, Death, and Myocardial Infarction Following Transcarotid Artery Revascularization vs Carotid Endarterectomy in Patients With Standard Surgical Risk. JAMA Neurol. 2023;80(5):437-444.
- Chaturvedi S. Transcarotid Artery Revascularization for Stroke Prevention-Multiple Elephants in the Room. JAMA Neurol. 2023;80(5):435-436
- Malas MB, Dakour-Aridi H, Kashyap VS, Eldrup-Jorgensen J, Wang GJ, Motaganahalli RL, Cronenwett JL, Schermerhorn ML. TransCarotid Revascularization With Dynamic Flow Reversal Versus Carotid Endarterectomy in the Vascular Quality Initiative Surveillance Project. Ann Surg. 2022;276:398-403.
- Perez-Troncoso D, Epstein D, Davies AH, Thapar A. Cost-effectiveness of carotid endarterectomy in symptomatic patients. Brit J Surgy. 2023;110:193-199.
- Halliday A, Mansfield A, Marro J, Peto C, Peto R, Potter J, Thomas D. Prevention of disabling and fatal strokes by successful carotid endarterectomy in patients without recent neurological symptoms: randomised controlled trial. Lancet. 2004;363:1491-1502.
- Rothwell PM, Eliasziw M, Gutnikov SA, Warlow CP, Barnett HJ. Sex difference in the effect of time from symptoms to surgery on benefit from carotid endarterectomy for transient ischemic attack and nondisabling stroke. Stroke. 2004;35:2855-2861.
- Bekelis K, Skinner J, Gottlieb D, Goodney P. De-adoption and exnovation in the use of carotid revascularization: retrospective cohort study. BMJ. 2017;359:j4695.
- Rothwell PM, Eliasziw M, Gutnikov SA, Fox AJ, Taylor DW, Mayberg MR, Warlow CP, Barnett HJ. Analysis of pooled data from the randomised controlled trials of endarterectomy for symptomatic carotid stenosis. Lancet. 2003;361:107-116.
- Beach KW, Leotta DF, Zierler RE. Carotid Doppler velocity measurements and anatomic stenosis: correlation is futile. Vasc Endovascular Surg. 2012;46:466-474.
- Gornik HL, Rundek T, Gardener H, Benenati JF, Dahiya N, Hamburg NM, Kupinski AM, Leers SA, Lilly MP, Lohr JM, et al. Optimization of duplex velocity criteria for diagnosis of internal carotid artery (ICA) stenosis: A report of the Intersocietal Accreditation Commission (IAC) Vascular Testing Division Carotid Diagnostic Criteria Committee. Vasc Med. 2021;26:515-525.
- Townsend TC, Saloner D, Pan XM, Rapp JH. Contrast material-enhanced MRA overestimates severity of carotid stenosis, compared with 3D time-of-flight MRA. J Vasc Surg. 2003;38:36-40.
- Horev A, Honig A, Cohen JE, Goldbart A, Dizitzer Y, Star M, Gomori JM, Zlotnik Y, Ifergane G, Borodetsky V, et al. Overestimation of carotid stenosis on CTA – Real world experience. J Clin Neurosci. 2021;85:36-40.
- Jareczek F, Farrell MB, E. L, Sila CA, Terry J, Sacks D, Kalapos P, Simon S, Crockroft KM. Over estimation of percent stenosis by physician operators may lead to carotid stent over utilisation. Available at: https://www.ahajournals.org/doi/10.1161/str.54.suppl_1.133. 2023 International/conference oral abstract #133. 2023.
- Saba L, Saam T, Jager HR, Yuan C, Hatsukami TS, Saloner D, Wasserman BA, Bonati LH, Wintermark M. Imaging biomarkers of vulnerable carotid plaques for stroke risk prediction and their potential clinical implications. Lancet Neurol. 2019;18:559-572.
- van Dam-Nolen DHK, Truijman MTB, van der Kolk AG, Liem MI, Schreuder F, Boersma E, Daemen M, Mess WH, van Oostenbrugge RJ, van der Steen AFW, et al. Carotid Plaque Characteristics Predict Recurrent Ischemic Stroke and TIA: The PARISK (Plaque At RISK) Study. JACC Cardiovascular Imaging. 2022;15:1715-1726.
- Abbott AL. Imaging the Carotid Artery: Counterpoint-Why Luminal Stenosis Remains the Most Important Imaging Feature in 2022. American Journal of Roentgenology. 2023;220:345-346.
- Eckstein HH, Reiff T, Ringleb P, Jansen O, Mansmann U, Hacke W, Investigators S. SPACE-2: A Missed Opportunity to Compare Carotid Endarterectomy, Carotid Stenting, and Best Medical Treatment in Patients with Asymptomatic Carotid Stenoses. Eur J Vasc Endovasc Surg. 2016;51:761-765.
- 2023 Annual Report of the Boards of Trustees of the Federal Hospital Insurance and Federal Supplementary Medical Insurance Trust Funds, 2023: https://www.cms.gov/oact/tr/2023.
- Kruk ME, Gage AD, Arsenault C, Jordan K, Leslie HH, Roder-DeWan S, Adeyi O, Barker P, Daelmans B, Doubova SV, et al. High-quality health systems in the Sustainable Development Goals era: time for a revolution. Lancet Glob Health. 2018;6:e1196-e1252.
- The Council of Canadian Academies (CCA). Fault Lines. Ottawa (ON): Expert Panel on the Socioeconomic Impacts of Science and Health Misinformation, CCA, 2023. Available at: https://cca-reports.ca/reports/the-socioeconomic-impacts-of-health-and-science-misinformation/.
- Berwick DM. Salve Lucrum: The Existential Threat of Greed in US Health Care. JAMA 2023;329:629-630.
- Venermo M, Wang G, Sedrakyan A, Mao J, Eldrup N, DeMartino R, Mani K, Altreuther M, Beiles B, Menyhei G, et al. Editor’s Choice – Carotid Stenosis Treatment: Variation in International Practice Patterns. Eur J Vasc Endovasc Surg. 2017;53:511-519.
- Sorber R, Holscher CM, Zarkowsky DS, Abularrage CJ, Black JH, 3rd, Wang GJ, Hicks CW. Increased Regional Market Competition is Associated with a Lower Threshold for Revascularization in Asymptomatic Carotid Artery Stenosis. Annals Vasc Surg. 2022;87:164-173.
- Abbott AL, Budincevic H. Carotid stenosis management: Getting the genie back in the bottle. HJVEVS. 2023;4:84-85.
Anne Abbott is a neurologist in the Department of Neuroscience at Central Clinical School, Monash University, in Melbourne, Australia. She has received several grants for independent research on the topic of stroke prevention. However, she was not academically funded at the time of creating this manuscript. She is also the founding member of FACTCAT. All authors, and most co-signatories, are FACTCAT members. The views of particular FACTCATs do not necessarily reflect the views of others. Lawrence Schott, MD, is a retired neuroradiologist, in Ireland and was the medical officer (2000–2013) for the Coverage and Analysis Group (CAG), Office of Clinical Standards and Quality (OCSQ), at CMS, and the lead medical officer, Medicare Evidence Development & Coverage Advisory Committee (MEDCAC), Management of Carotid Atherosclerosis, Jan. 25, 2012. Lan Gao, MMed, PhD, is a senior lecturer in health economics at the Institute for Health Transformation, School of Health & Social Development, Deakin University, in Melbourne, Australia. Hrvoje Budincevic, MD, PhD, is head of the Stroke and Intensive Care Unit and deputy dead of the Department of Neurology at Sveti Duh University Hospital, in Zagreb, Croatia. Rishad Faruqi, MD, is clinical associate professor of surgery (affiliate) at Stanford University and clinical associate professor of surgery (affiliate) at University of California, San Francisco (UCSF). Tatjana Rundek, MD, PhD, is professor of neurology in the Department of Neurology at Miller School of Medicine in Miami, Florida. She holds National Institutes of Health (NIH) grants that are unrelated to this manuscript. Jean-Baptiste Ricco, MD, PhD, is a vascular surgeon in the Department of Vascular Surgery at the University Hospital of Toulouse and University of Poitiers in France. Saeid Shahidi, MD, is a senior consultant in vascular surgery, chief consultant in supra aortic disease, research chief physician in vascular surgery, and associate professor at the University of Copenhagen and Zealand, Department of Cardiology and Vascular Surgery at Zealand University Hospital in Roskilde, Denmark. Gert J. de Borst, MD, PhD, is head of the Department of Vascular Surgery at University Medical Centre of Utrecht, the Netherlands.
Co-signatories: Anne Abbott (Neurologist, Australia); Mohamad AbdalKader (interventional neuroradiologist, U.S.); Yogesh Acharya (vascular surgeon, Ireland); Nishath Altaf (vascular surgeon, Australia); Pier Luigi Antignani (angiologist, Italy); Omar Ayoub (neurologist, Saudi Arabia); Hernan Bazan (vascular surgeon, U.S.); Peter Bell (vascular surgeon, United Kingdom); Ruth Benson (vascular surgeon, New Zealand); Aleš Blinc (cardiologist/vascular physician, Slovenia); Alejandro Brunser (neurologist, Chile); Hrvoje Budincevic (neurologist, Croatia); Jonathan Cardella (vascular surgeon, U.S.); Robert Chang (vascular surgeon, U.S.); Alun Davies (vascular surgeon, United Kingdom); Gert J. de Borst (vascular surgeon, the Netherlands); Rishad Faruqi (vascular surgeon, U.S.); Aaron Gaekwad (neurologist physician trainee, Australia); Lan Gao (health economist, Australia); Hannah Gardener (epidemiologist, U.S.); Richard Genova (registered vascular technologist, U.S.); George Geroulakos (vascular surgeon, Greece/United Kingdom); Athanasios Giannoukas (vascular surgeon, Greece); Harry Gibbs (general & vascular medicine physician, Australia); Peter Gloviczki, (vascular surgeon, U.S.); Anders Gottsäter (vascular physician, Sweden); Guillaume Goudot (vascular physician, France); Jose Gutierrez (neurologist, U.S.); Vassilis Hadjianastassiou (vascular surgeon, United Kingdom); Kimberley Hammar (PhD candidate & resident [emergency medicine], Sweden); Robert Harbaugh (neurosurgeon, U.S.); Eitan Heldenberg (vascular surgeon, Israel); Caitlin Hicks (vascular surgeon, U.S.); Michal Juszynski (vascular surgeon, Poland); Stavros Kakkos (vascular surgeon, Greece); Anthony Kam (neuroradiologist, Australia); Zubair Kareem (neurologist, U.S.); Tien Khoo (internist [general & acute] medicine, Australia); Stefan Kiechl (neurologist, Austria); Timothy Kleinig (neurologist, Australia); Michael Knoflach (neurologist, Austria); Simona Lattanzi (neurologist, Italy); Kaitlyn Lillemoe (neurologist, U.S.); Ian Loftus (vascular surgeon, United Kingdom); Fedor Lurie (vascular surgeon, U.S.); Jonas Malmstedt (vascular surgeon, Sweden); Devender Mittapalli (vascular surgeon, United Kingdom); Wesley Moore (vascular surgeon, U.S.); Bibombe Patrice Mwipatayi (vascular surgeon, Australia); Achim Neufang (vascular surgeon, Germany); Branimir Nevajda (neurologist and stroke specialist, United Kingdom); Alexander Oberhuber (vascular surgeon/endovascular specialist, Germany); Jean Panneton (vascular surgeon, U.S.); Malay Patel (vascular surgeon, India); David Pelz (neuroradiologist, Canada); Fernando Picazo Pineda (vascular surgeon, Australia); Bartlomiej Piechowski-Jozwiak (neurologist, United Arab Emirates); Holger Poppert (neurologist, Germany); Alkoredi Fatai Radji (neurologist, France); Jean-Baptiste Ricco (vascular surgeon, France); Peter Ringleb (neurologist, Germany); Jenni Robertson (consumer representative & stroke survivor, United Kingdom); David Robinson (vascular surgeon, Australia); Sara Rostanski (vascular neurologist, U.S.); Tatjana Rundek (neurologist, U.S.); David Saloner (arterial/biomedical imaging specialist, U.S.); Felix Schlachetzki (neurologist, Germany); Lawrence Schott (neuroradiologist, Ireland); Saeid Shahidi (vascular surgeon, Denmark); Joseph Shalhoub (vascular surgeon, United Kingdom); Francesco Spinelli (vascular surgeon, Italy); Daniel Staub (angiologist, Switzerland); Francesco Stilo (vascular surgeon, Italy); Tim Stokes (consumer representative & survivor of stroke misdiagnosis, Australia); Sherif Sultan (vascular surgeon, Ireland); Costas Thomopoulos (cardiologist, Greece); Raffi Topakian (Neurologist, Austria); Francesco Torella (vascular surgeon, United Kingdom); Olise Uyagu (general medical practitioner, Australia); Claude Vaislic (vascular surgeon, France); Fred Weaver (vascular surgeon, U.S.); Martin Veller (vascular surgeon, South Africa); Maarit Venermo (vascular surgeon, Finland); Alex Vesey (vascular surgeon, United Kingdom); Tissa Wijeratne (neurologist, Australia); Joshua Willey (neurologist, U.S.); Mary-Ann Williams (consumer representative & stroke survivor, Australia); Roz Williamson (allied health, Australia); Kim Wootton (consumer representative & stroke survivor, Australia); and Wei Zhou (vascular surgeon, U.S.).


















 Froedtert Hospital has become the first hospital in Wisconsin to receive national verification as a Comprehensive Inpatient Vascular Center through the American College of Surgeons (ACS) Vascular Verification Program (VVP), in partnership with the SVS.
Froedtert Hospital has become the first hospital in Wisconsin to receive national verification as a Comprehensive Inpatient Vascular Center through the American College of Surgeons (ACS) Vascular Verification Program (VVP), in partnership with the SVS.
 The Society for Vascular Surgery (SVS) Foundation’s 2025 annual report details a year of significant progress in research, education and advocacy aimed at improving vascular health worldwide.
The Society for Vascular Surgery (SVS) Foundation’s 2025 annual report details a year of significant progress in research, education and advocacy aimed at improving vascular health worldwide.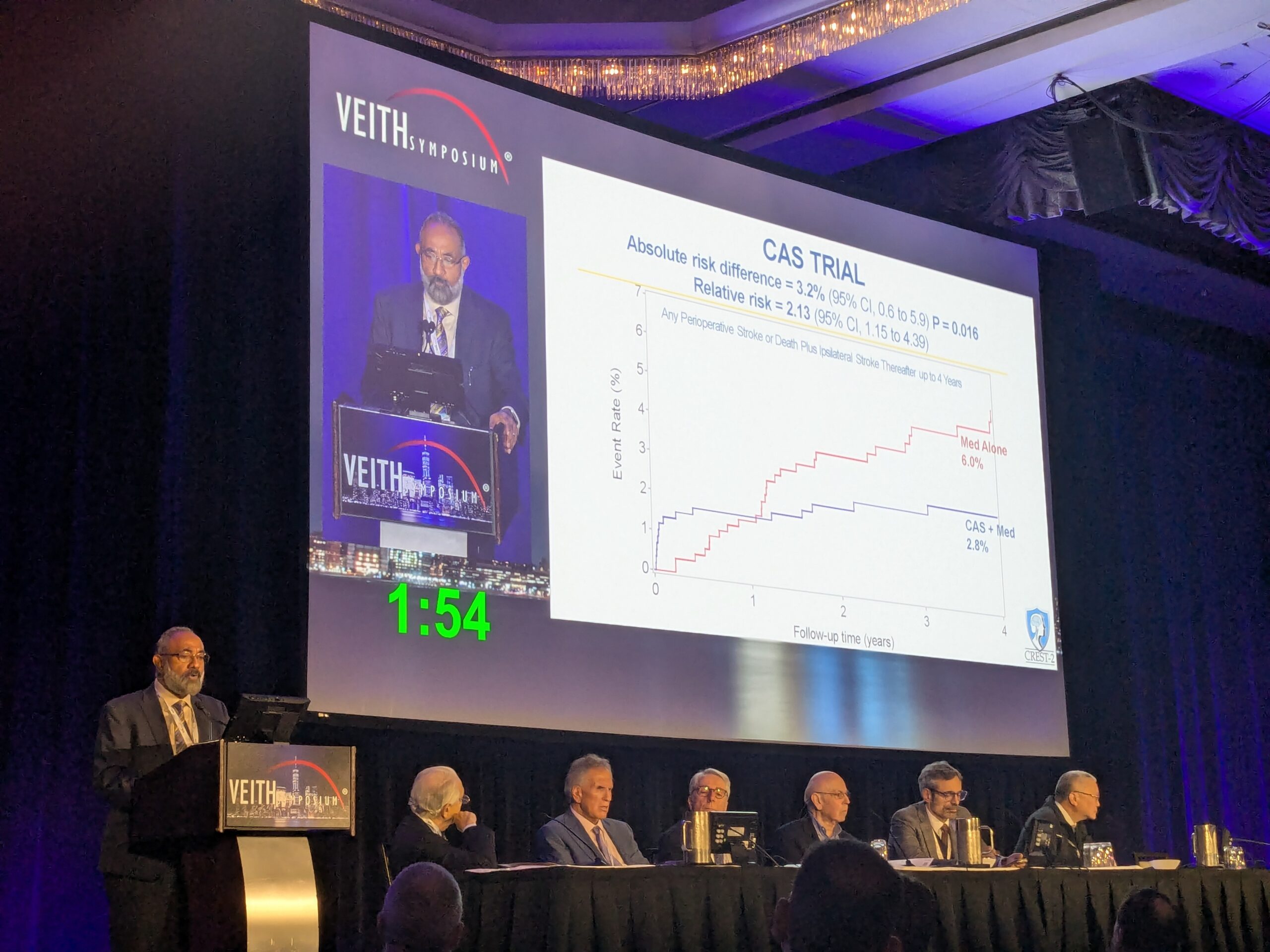











 Three vascular surgeons discuss how and when they deploy the transformative
Three vascular surgeons discuss how and when they deploy the transformative 


 Elucid has announced the launch of its PlaqueIQ image analysis software for the quantification and classification of plaque morphology in the
Elucid has announced the launch of its PlaqueIQ image analysis software for the quantification and classification of plaque morphology in the 



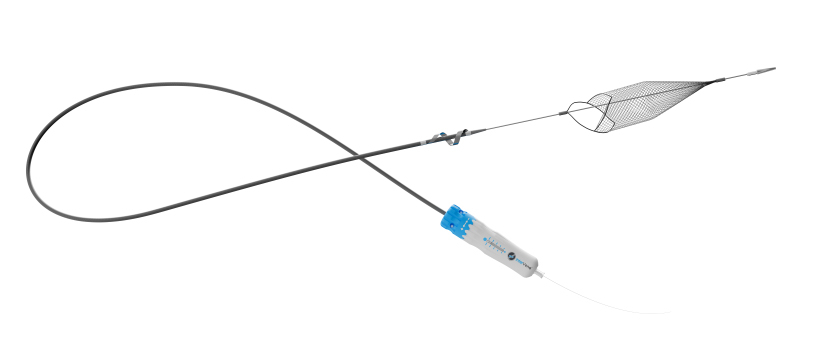
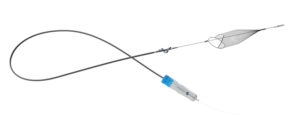



 Proposals for the new “Hot Topics” session at the
Proposals for the new “Hot Topics” session at the 





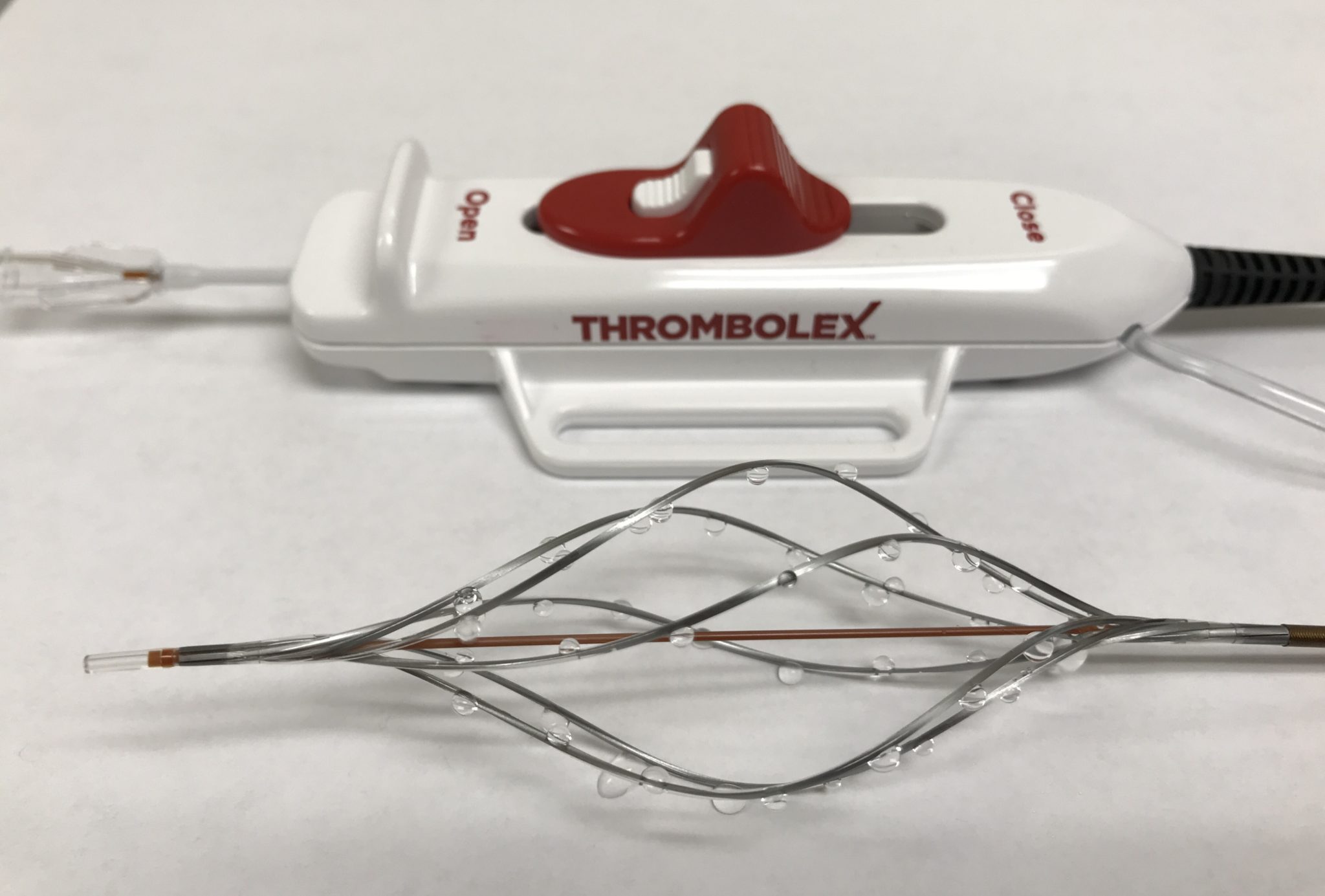





 New findings chronicling progress with both the
New findings chronicling progress with both the 
















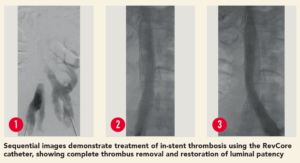 The patient was a 35-year-old male who presented with worsening back pain, leg swelling and edema that on imaging appeared to point to an acute DVT. Several years prior, he had undergone thrombectomy, IVC stenting and a bilateral iliocaval reconstruction for chronic IVC occlusion and associated iliofemoral thrombosis. The IVC and common iliac vein (CIV) stents were thrombosed.
The patient was a 35-year-old male who presented with worsening back pain, leg swelling and edema that on imaging appeared to point to an acute DVT. Several years prior, he had undergone thrombectomy, IVC stenting and a bilateral iliocaval reconstruction for chronic IVC occlusion and associated iliofemoral thrombosis. The IVC and common iliac vein (CIV) stents were thrombosed.

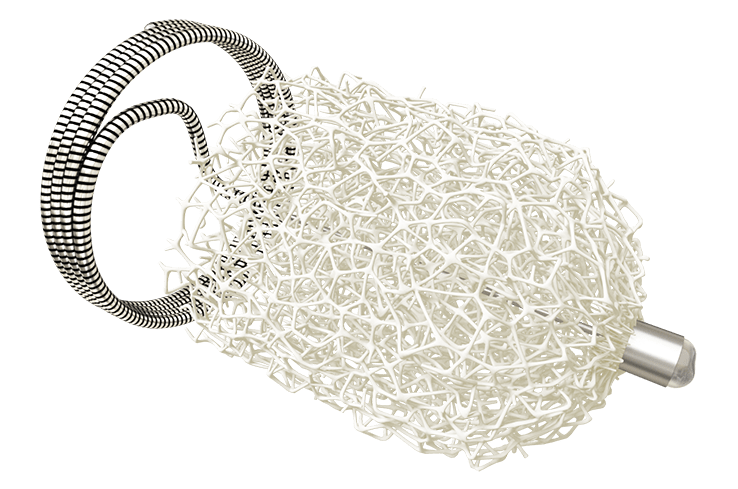






 Data from the AMDS PERSEVERE and PROTECT trials—both assessing the use of the Ascyrus medical dissection stent (AMDS, Artivion)—were presented in late-breaking science presentations at the 39th European Association for Cardio-Thoracic Surgery (EACTS) annual meeting (8–11 October, Copenhagen, Denmark).
Data from the AMDS PERSEVERE and PROTECT trials—both assessing the use of the Ascyrus medical dissection stent (AMDS, Artivion)—were presented in late-breaking science presentations at the 39th European Association for Cardio-Thoracic Surgery (EACTS) annual meeting (8–11 October, Copenhagen, Denmark).
 Nurea’s PRAEVAorta 2
Nurea’s PRAEVAorta 2 

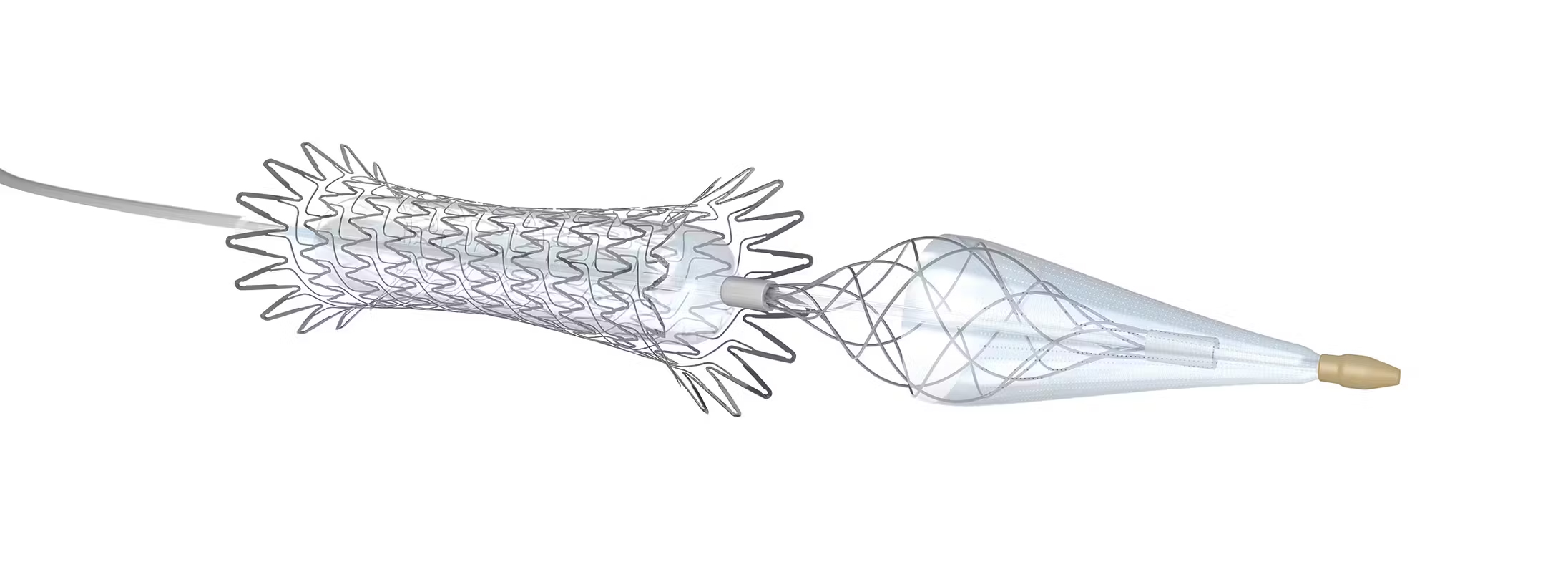
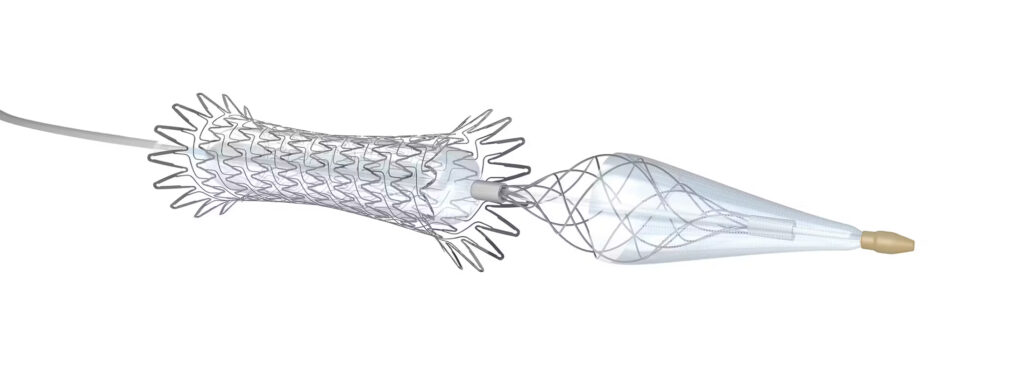
















 Teams formally defined as specializing in chronic limb-threatening ischemia (CLTI) were associated with a decreased risk of major
Teams formally defined as specializing in chronic limb-threatening ischemia (CLTI) were associated with a decreased risk of major 
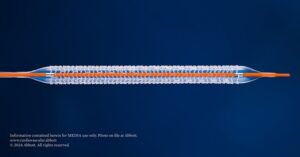











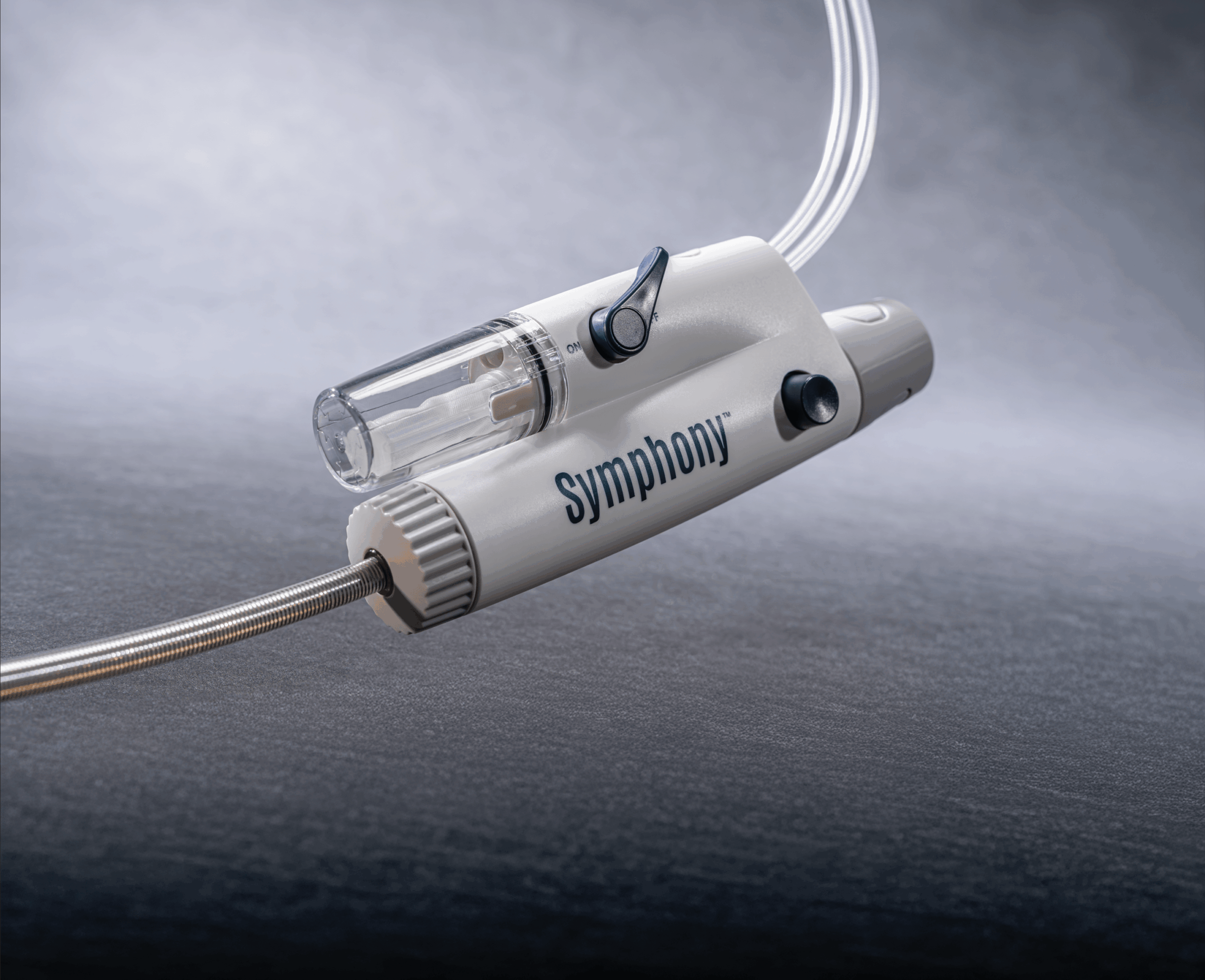
 Imperative Care has announced efficacy and safety results from the pivotal SYMPHONY-PE trial evaluating the company’s Symphony thrombectomy system in the treatment of acute pulmonary embolism (PE).
Imperative Care has announced efficacy and safety results from the pivotal SYMPHONY-PE trial evaluating the company’s Symphony thrombectomy system in the treatment of acute pulmonary embolism (PE).














 Microbot Medical today announced that the Food and Drug Administration (FDA) has granted 510(k) clearance for the Liberty system, the first FDA cleared single use, remotely operated robotic system for peripheral endovascular procedures, states the company in a recent press release.
Microbot Medical today announced that the Food and Drug Administration (FDA) has granted 510(k) clearance for the Liberty system, the first FDA cleared single use, remotely operated robotic system for peripheral endovascular procedures, states the company in a recent press release. 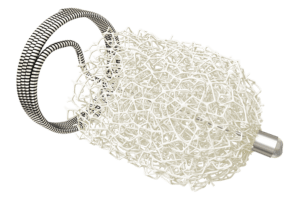












 Boston Scientific has recalled its Carotid Wallstent Monorail endoprosthesis owing to a “manufacturing defect” that has led to devices having an inner lumen that is smaller than specifications, causing resistance when withdrawing the stent delivery system.
Boston Scientific has recalled its Carotid Wallstent Monorail endoprosthesis owing to a “manufacturing defect” that has led to devices having an inner lumen that is smaller than specifications, causing resistance when withdrawing the stent delivery system.



 The speed with which
The speed with which 

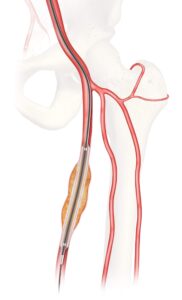
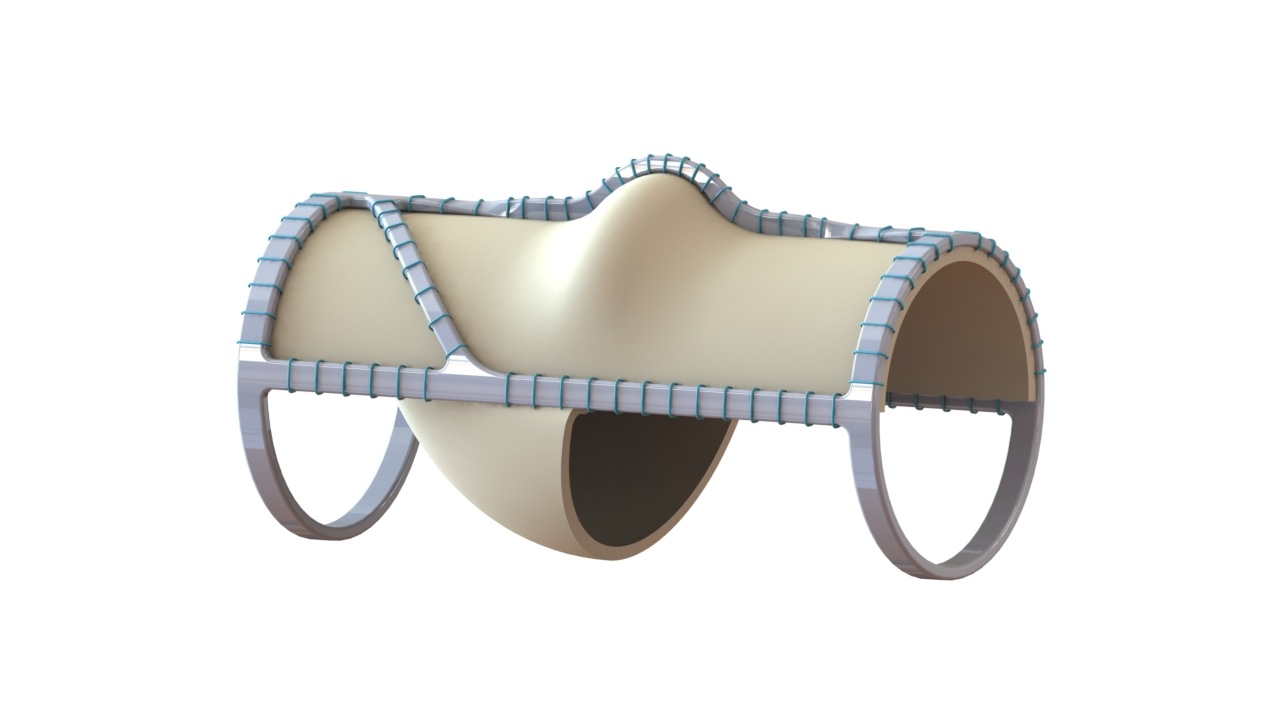











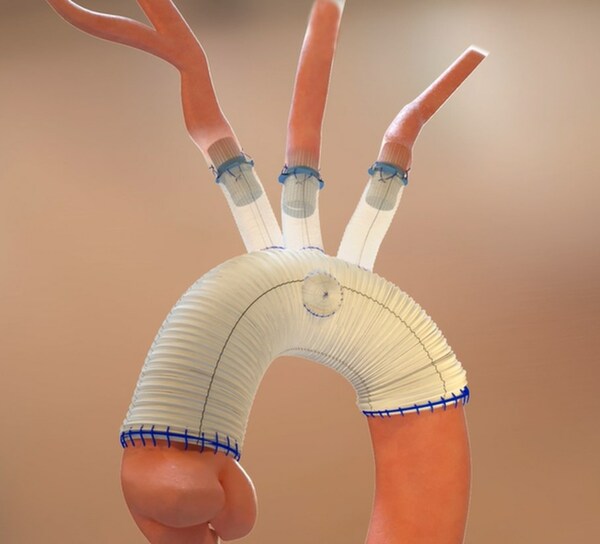
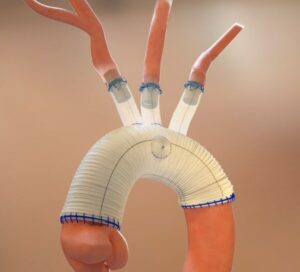














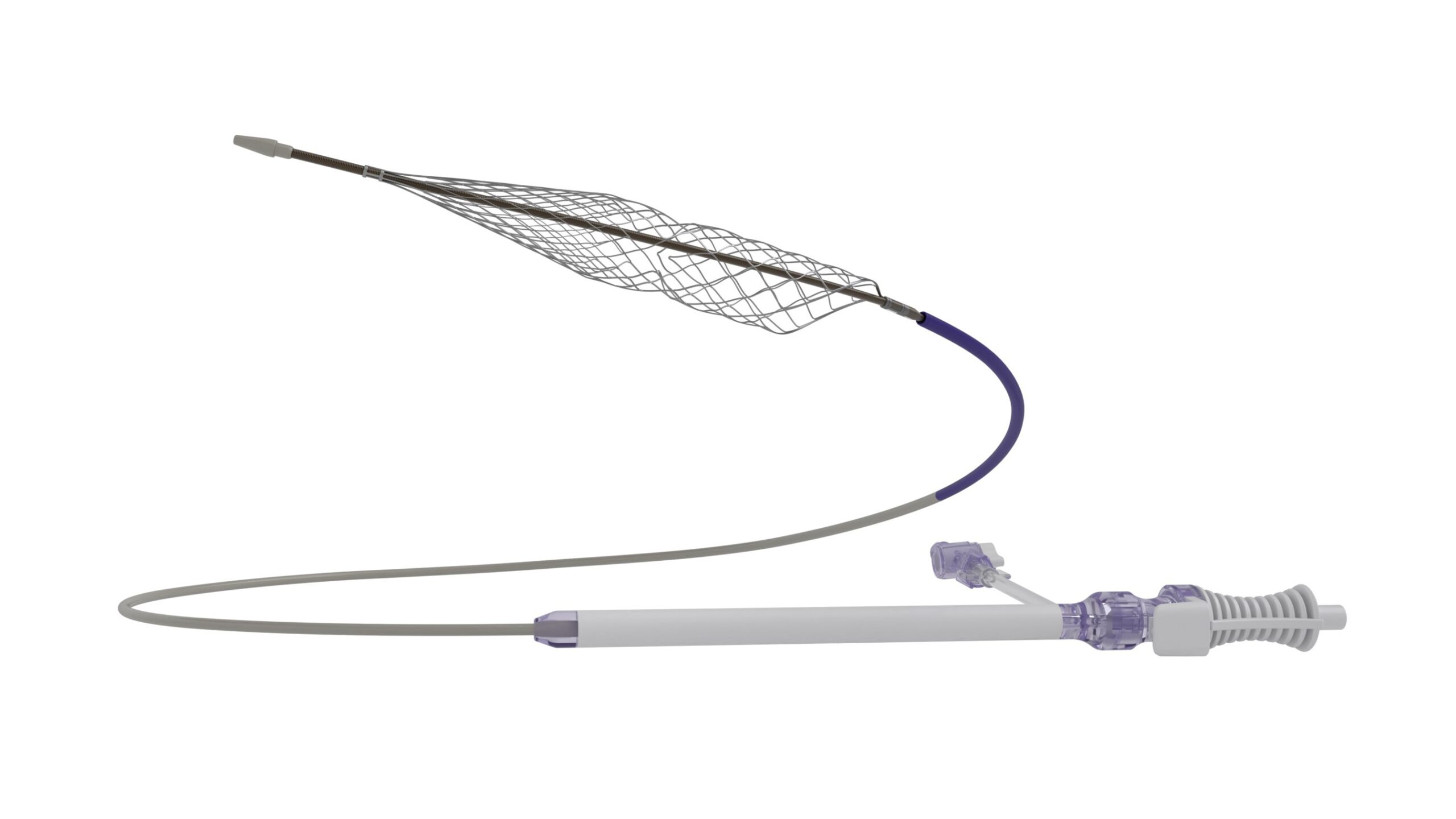
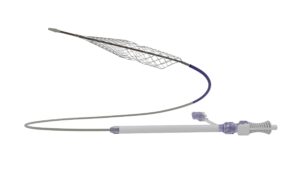































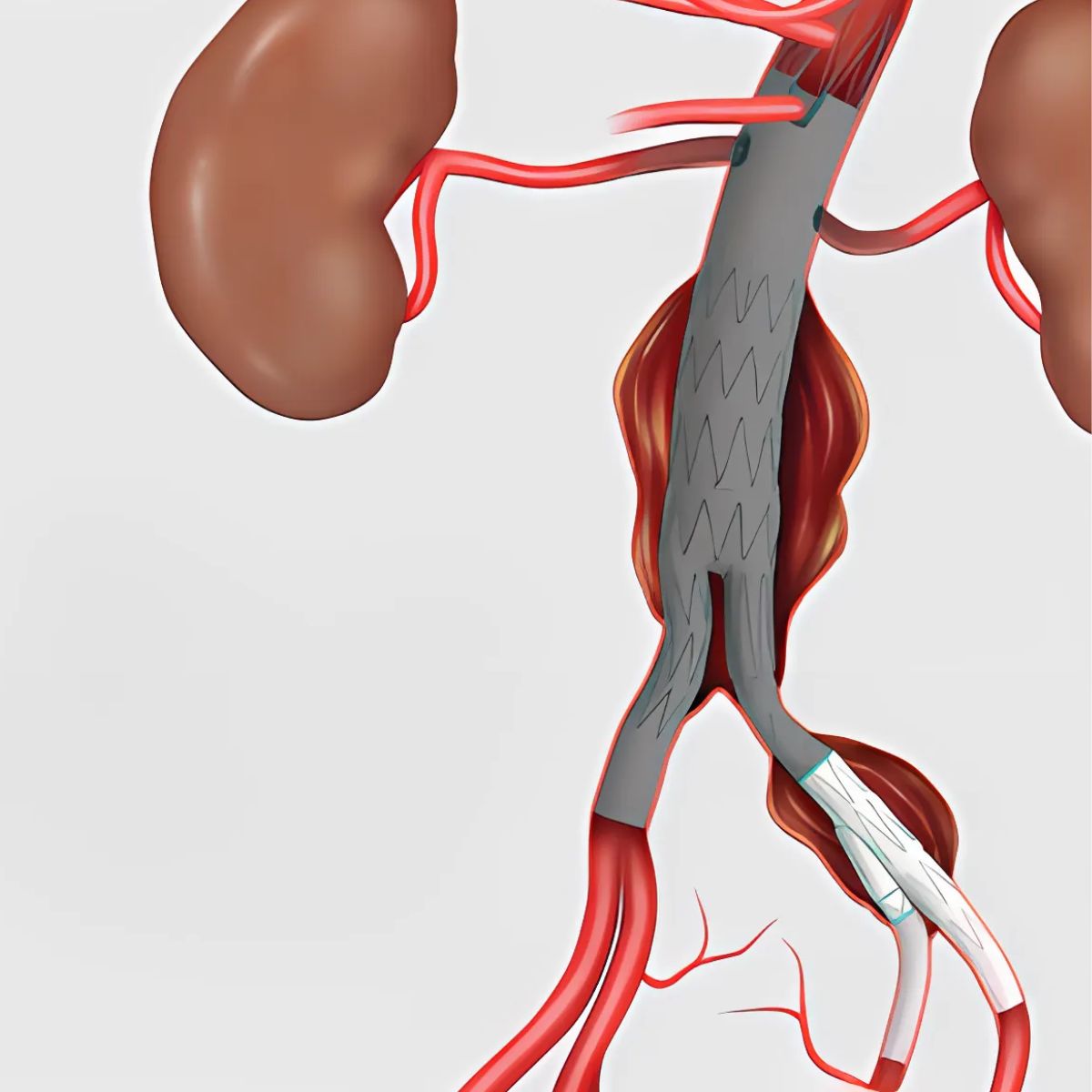
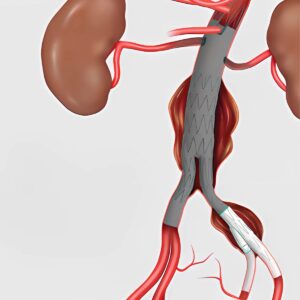
























 Results from the CLN-PRO-V007 pivotal phase 3 clinical trial of the acellular tissue engineered vessel (ATEV; Humacyte) in arteriovenous access for patients at high risk of autologous arteriovenous fistula (AVF) maturation failure with end-stage renal disease were presented at the 2025 Vascular Annual Meeting (VAM) in New Orleans (June 4–7).
Results from the CLN-PRO-V007 pivotal phase 3 clinical trial of the acellular tissue engineered vessel (ATEV; Humacyte) in arteriovenous access for patients at high risk of autologous arteriovenous fistula (AVF) maturation failure with end-stage renal disease were presented at the 2025 Vascular Annual Meeting (VAM) in New Orleans (June 4–7).
 Attendees of the
Attendees of the 











 InspireMD has been granted premarket approval (PMA) from the Food and Drug Administration (FDA) for its CGuard Prime
InspireMD has been granted premarket approval (PMA) from the Food and Drug Administration (FDA) for its CGuard Prime 





 Inquis Medical has announced that its Aventus thrombectomy system has received 510(k) clearance from the US Food and Drug Administration (FDA) for an expanded indication to treat pulmonary embolism (PE).
Inquis Medical has announced that its Aventus thrombectomy system has received 510(k) clearance from the US Food and Drug Administration (FDA) for an expanded indication to treat pulmonary embolism (PE).




 The Society for Vascular Surgery (SVS) has elected Andres Schanzer, MD, as the incoming vice president and Malachi G. Sheahan III, MD, as the new secretary, revealed during Friday night’s Annual Business Meeting, as part of the 2025 Vascular Annual Meeting (June 4-7) in New Orleans.
The Society for Vascular Surgery (SVS) has elected Andres Schanzer, MD, as the incoming vice president and Malachi G. Sheahan III, MD, as the new secretary, revealed during Friday night’s Annual Business Meeting, as part of the 2025 Vascular Annual Meeting (June 4-7) in New Orleans.














 Staged total endovascular aortic repair (TEAR) utilizing arch branched and thoracoabdominal fenestrated and branched endografts is effective, but identified predictors of morbidity and mortality—including stroke—highlight the importance of individualized risk assessment to optimize outcomes. These are some of the key findings of a study presented during yesterday’s Plenary Session 3.
Staged total endovascular aortic repair (TEAR) utilizing arch branched and thoracoabdominal fenestrated and branched endografts is effective, but identified predictors of morbidity and mortality—including stroke—highlight the importance of individualized risk assessment to optimize outcomes. These are some of the key findings of a study presented during yesterday’s Plenary Session 3. 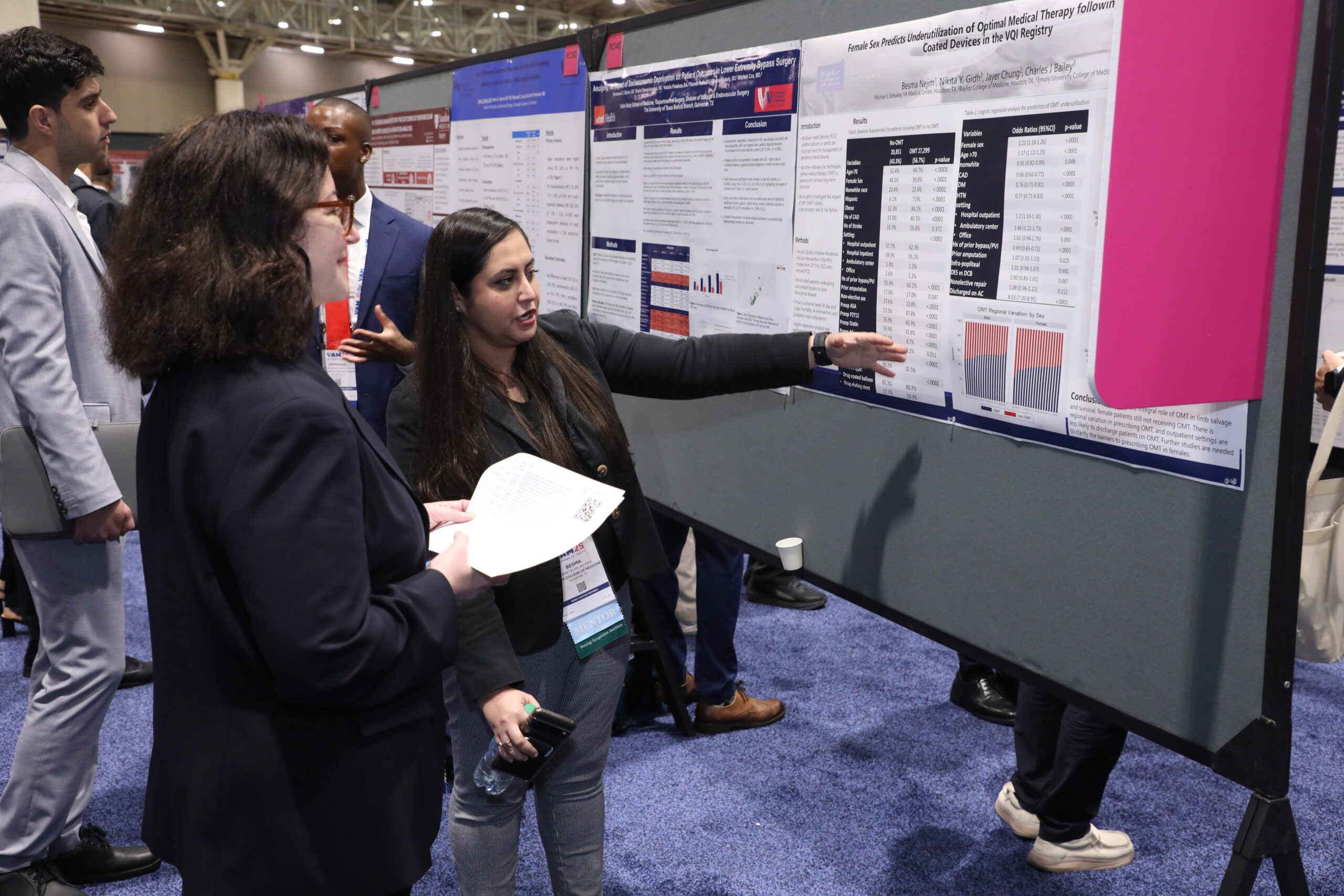







 SVS members in good standing can still
SVS members in good standing can still 






















































 The
The 




 How a group of
How a group of 











 Imperative Care has announced the completion of patient enrollment in its SYMPHONY-PE study, a pivotal investigational device exemption (IDE) trial evaluating the safety and efficacy of the company’s Symphony thrombectomy system for the treatment of acute pulmonary embolism (PE).
Imperative Care has announced the completion of patient enrollment in its SYMPHONY-PE study, a pivotal investigational device exemption (IDE) trial evaluating the safety and efficacy of the company’s Symphony thrombectomy system for the treatment of acute pulmonary embolism (PE).






























 Twelve-month outcomes of the VenaSeal Spectrum venous leg ulcer (VLU) trial, assessing time to ulcer healing following treatment with the VenaSeal (Medtronic) closure system, have demonstrated an 81.3% ulcer healing rate at one year.
Twelve-month outcomes of the VenaSeal Spectrum venous leg ulcer (VLU) trial, assessing time to ulcer healing following treatment with the VenaSeal (Medtronic) closure system, have demonstrated an 81.3% ulcer healing rate at one year.
 Results of the ACCESS 2 study, evaluating arteriovenous fistula (AVF) outcomes following use of the Sirogen (Vascular Therapies) sirolimus-eluting collagen implant, have shown that the device failed to meet non-inferiority for clinical fistula maturation compared to control at six months.
Results of the ACCESS 2 study, evaluating arteriovenous fistula (AVF) outcomes following use of the Sirogen (Vascular Therapies) sirolimus-eluting collagen implant, have shown that the device failed to meet non-inferiority for clinical fistula maturation compared to control at six months.













 T
T






 T
T

















 Merit Medical Systems has announced that the six-month results from the randomized arm of the Wrapsody arteriovenous access efficacy—WAVE—trial are scheduled for publication in the April issue of Kidney International, ahead of the presentation of 12-month results from the trial at the Society of Interventional Radiology’s 50th annual scientific meeting (March 29–April 2) in Nashville, Tennessee.
Merit Medical Systems has announced that the six-month results from the randomized arm of the Wrapsody arteriovenous access efficacy—WAVE—trial are scheduled for publication in the April issue of Kidney International, ahead of the presentation of 12-month results from the trial at the Society of Interventional Radiology’s 50th annual scientific meeting (March 29–April 2) in Nashville, Tennessee.
 The importance of
The importance of 
 The Society for Vascular Surgery (SVS) has developed a comprehensive
The Society for Vascular Surgery (SVS) has developed a comprehensive 











 Janet Powell, the clinical trial investigator who played a pivotal role in several landmark abdominal aortic aneurysm (AAA) studies, has died at the age of 79. At the time of her death, Powell was professor of vascular biology and medicine at Imperial College London (London, UK).
Janet Powell, the clinical trial investigator who played a pivotal role in several landmark abdominal aortic aneurysm (AAA) studies, has died at the age of 79. At the time of her death, Powell was professor of vascular biology and medicine at Imperial College London (London, UK). 


 “One of her great contributions was to ask the questions of research that others sometimes feared to ask. More often however, others just didn’t think of these questions. She always saw others’ work through a different and often probing eye, but always with an inquisitive perspective.
“One of her great contributions was to ask the questions of research that others sometimes feared to ask. More often however, others just didn’t think of these questions. She always saw others’ work through a different and often probing eye, but always with an inquisitive perspective.
 As the new administration headed up by President Donald Trump gets underway, work has begun on his vision for a fundamental transformation of the federal government, including some aspects of the
As the new administration headed up by President Donald Trump gets underway, work has begun on his vision for a fundamental transformation of the federal government, including some aspects of the 





 Philips has recently announced that it will no longer sell its Tack endovascular system in the U.S. following
Philips has recently announced that it will no longer sell its Tack endovascular system in the U.S. following 



 Stereotaxis has announced a Food and Drug Administration (FDA) regulatory submission for the first robotically navigated catheter designed to expand usage of robotic magnetic navigation into the broader endovascular field.
Stereotaxis has announced a Food and Drug Administration (FDA) regulatory submission for the first robotically navigated catheter designed to expand usage of robotic magnetic navigation into the broader endovascular field.











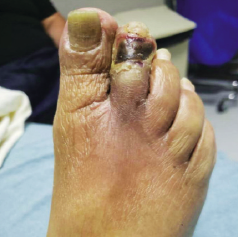
 “This patient, a 68-year-old male with diabetes and end-stage kidney disease [ESKD], is absolutely someone who would have been amputated, but on whom we carried out a deep-vein arterialization [DVA] procedure, allowing blood flow to get to the foot,” she says. “That process takes time, and, during that time, you don’t want the wound to disintegrate, get infected and the person end up getting amputated. This being a patient with diabetes and ESKD, we are talking about the worst of the worst type of blood vessels. In spite of that, we healed this wound with, of course, great wound care: good blood flow was formed from the DVA, and then came TWO2 therapy.”
“This patient, a 68-year-old male with diabetes and end-stage kidney disease [ESKD], is absolutely someone who would have been amputated, but on whom we carried out a deep-vein arterialization [DVA] procedure, allowing blood flow to get to the foot,” she says. “That process takes time, and, during that time, you don’t want the wound to disintegrate, get infected and the person end up getting amputated. This being a patient with diabetes and ESKD, we are talking about the worst of the worst type of blood vessels. In spite of that, we healed this wound with, of course, great wound care: good blood flow was formed from the DVA, and then came TWO2 therapy.”
























 The
The 







 Akura Medical
Akura Medical









 Physicians are facing a fifth consecutive year of Medicare payment reductions due to policy adjustments and budget neutrality requirements in the Medicare Physician Fee Schedule. To address the issue, the SVS, in collaboration with congressional leaders and other physician organizations,
Physicians are facing a fifth consecutive year of Medicare payment reductions due to policy adjustments and budget neutrality requirements in the Medicare Physician Fee Schedule. To address the issue, the SVS, in collaboration with congressional leaders and other physician organizations, 
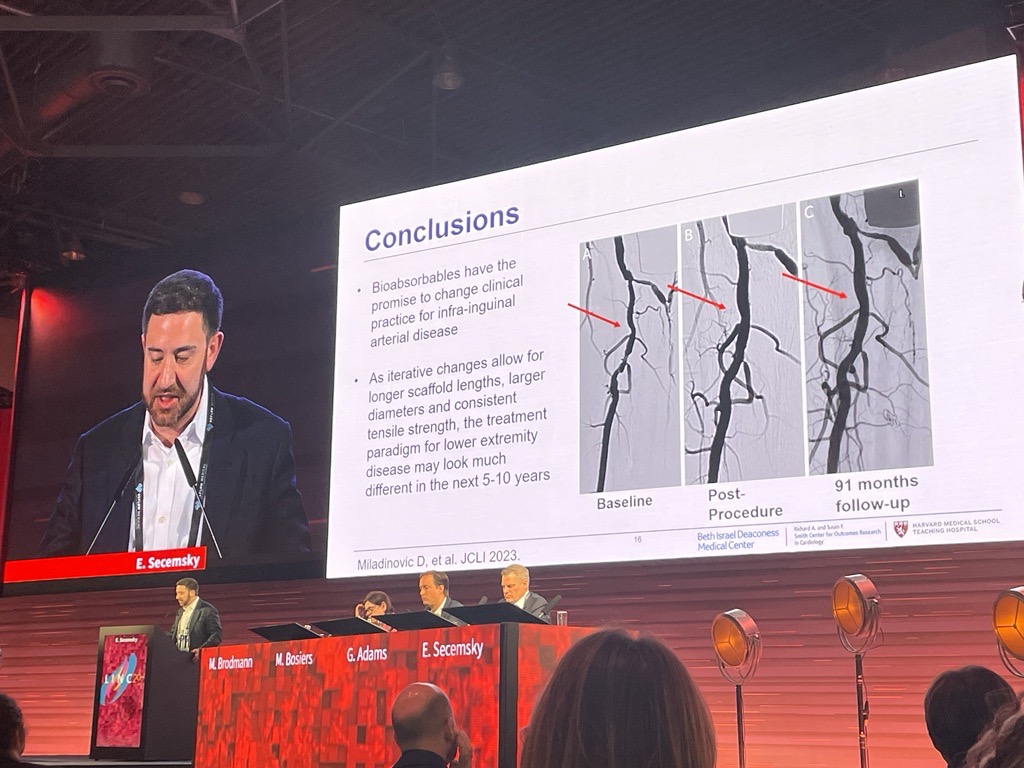











 Five prominent vascular surgeons have been named “at-large” members of the
Five prominent vascular surgeons have been named “at-large” members of the  Yazan Duwayri
Yazan Duwayri  Katherine Gallagher
Katherine Gallagher  Vikram Kashyap
Vikram Kashyap  Robert Molnar
Robert Molnar 
 The
The 

 Gore has announced recent CE mark of a lower profile Viabahn VBX balloon expandable endoprosthesis (VBX stent graft).
Gore has announced recent CE mark of a lower profile Viabahn VBX balloon expandable endoprosthesis (VBX stent graft).






















 A man in his late 60s with a left iliac vein stent and revision two years prior was referred for consultation. The patient discontinued his anticoagulation two months before he was referred for consultation for severe left leg swelling. Duplex ultrasound confirmed a reoccluded left iliac venous stent. The decision was made to intervene with mechanical thrombectomy.
A man in his late 60s with a left iliac vein stent and revision two years prior was referred for consultation. The patient discontinued his anticoagulation two months before he was referred for consultation for severe left leg swelling. Duplex ultrasound confirmed a reoccluded left iliac venous stent. The decision was made to intervene with mechanical thrombectomy.





















 Post election and with the end of the year on the horizon, a crucial but often overlooked period in the legislative calendar begins: the lame duck congressional session. This period, which occurs between the November elections and the start of the new
Post election and with the end of the year on the horizon, a crucial but often overlooked period in the legislative calendar begins: the lame duck congressional session. This period, which occurs between the November elections and the start of the new 



















 Sherazuddin Qureshi, MD, is a clinical assistant professor of surgery at Northwestern Medicine, Ryan Meyer, MD, is a vascular surgeon at RWJBarnabas Health, Erica Leith Mitchell, MD, is a professor of surgery at the University of Tennessee Health Science Center, Nicolas Mouawad, MD, is chief of vascular surgery at McLaren Bay region, and Issam Koleilat, MD, is also vascular surgeon at RWJBarnabas Health.
Sherazuddin Qureshi, MD, is a clinical assistant professor of surgery at Northwestern Medicine, Ryan Meyer, MD, is a vascular surgeon at RWJBarnabas Health, Erica Leith Mitchell, MD, is a professor of surgery at the University of Tennessee Health Science Center, Nicolas Mouawad, MD, is chief of vascular surgery at McLaren Bay region, and Issam Koleilat, MD, is also vascular surgeon at RWJBarnabas Health.















 Introduction
Introduction 






 The sixth edition of the Vascular Education and Self-Assessment Program (
The sixth edition of the Vascular Education and Self-Assessment Program (


 The Journal of Vascular Surgery-Cases, Innovations and Techniques (
The Journal of Vascular Surgery-Cases, Innovations and Techniques (
 On Tuesday, Sept. 24, The Society for Vascular Nursing (
On Tuesday, Sept. 24, The Society for Vascular Nursing (
 A webinar on “Perioperative Care in Open Aortic Vascular Surgery: Recommendations from
A webinar on “Perioperative Care in Open Aortic Vascular Surgery: Recommendations from 




 Abstracts for the 2025 Vascular Research Initiatives Conference (
Abstracts for the 2025 Vascular Research Initiatives Conference (
 Brajesh K. Lal, MD, assumed the Eastern Vascular Society (
Brajesh K. Lal, MD, assumed the Eastern Vascular Society (


 Findings from a retrospective analysis delivered at the recent European Society for Vascular Surgery (
Findings from a retrospective analysis delivered at the recent European Society for Vascular Surgery (
 In October, the most read stories from Vascular Specialist included a new leadership announcement from the American College of Surgeons (ACS); study results examining a potential link between strenuous physical activity and increased venous thromboembolism (VTE) risk in men; the publication of a new ebook to enhance vascular surgery education from The Association of Program Directors in Vascular Surgery (APDVS), and several more.
In October, the most read stories from Vascular Specialist included a new leadership announcement from the American College of Surgeons (ACS); study results examining a potential link between strenuous physical activity and increased venous thromboembolism (VTE) risk in men; the publication of a new ebook to enhance vascular surgery education from The Association of Program Directors in Vascular Surgery (APDVS), and several more.









 Royal Philips has announced enrolment of the first patient in the US THOR IDE clinical trial, which will study an innovative combined laser atherectomy and intravascular lithotripsy (IVL) catheter.
Royal Philips has announced enrolment of the first patient in the US THOR IDE clinical trial, which will study an innovative combined laser atherectomy and intravascular lithotripsy (IVL) catheter.

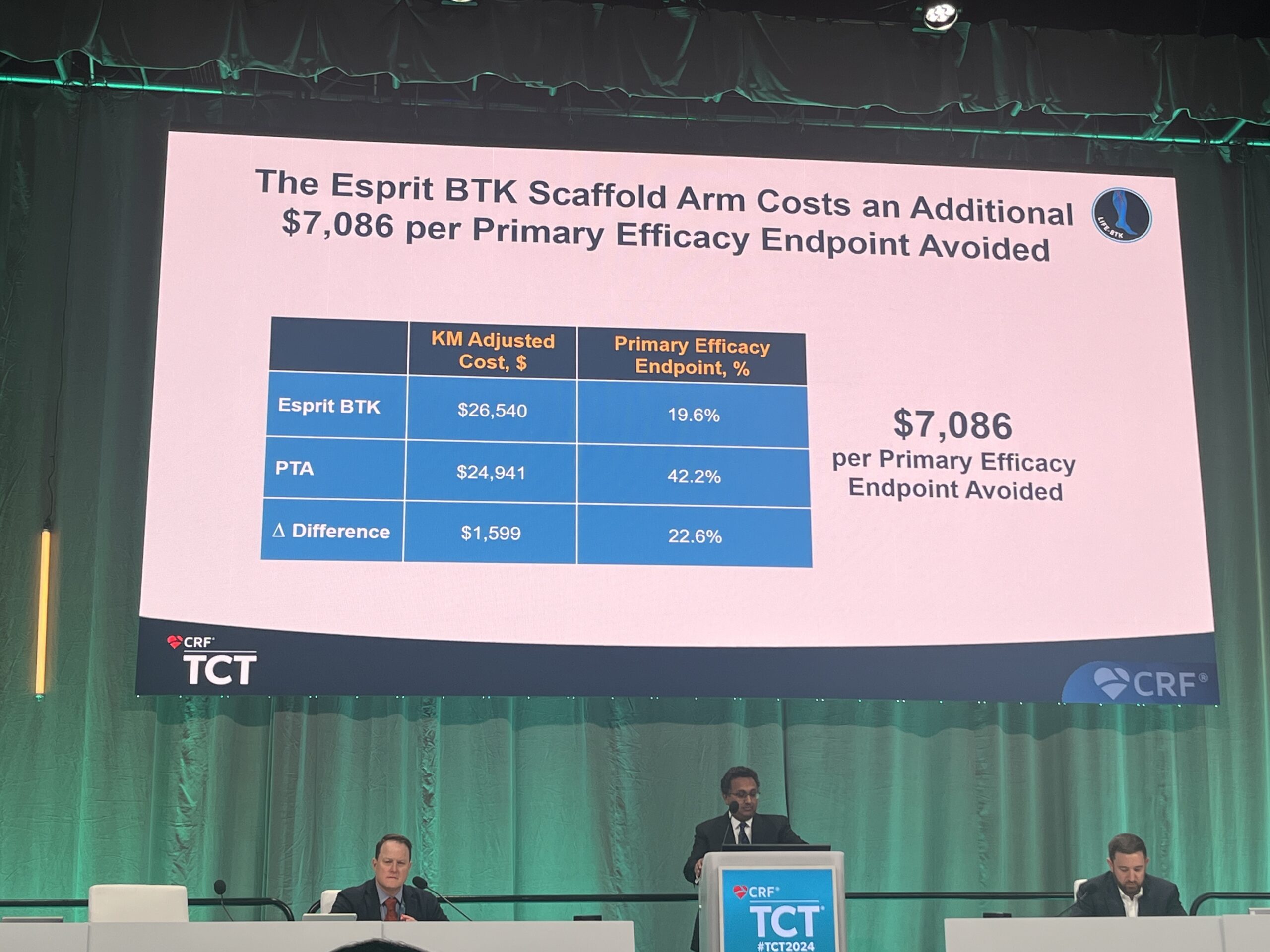

 The
The 



























 The
The 
 The Society for Vascular Surgery (SVS) online learning management system (LMS), now known as SVS VascuLEARN. The platform was formerly called
The Society for Vascular Surgery (SVS) online learning management system (LMS), now known as SVS VascuLEARN. The platform was formerly called 
















 Vince Weaver highlights a case in which the thrombus in the left superficial femoral artery (SFA) is tackled.
Vince Weaver highlights a case in which the thrombus in the left superficial femoral artery (SFA) is tackled.  Lucas Ferrer Cardona presents a case of complete thrombus in below-the-knee arteries.
Lucas Ferrer Cardona presents a case of complete thrombus in below-the-knee arteries.  Christopher Leville demonstrates the use of Pounce LP in the upper extremity.
Christopher Leville demonstrates the use of Pounce LP in the upper extremity. 







 VenaCore case report 2
VenaCore case report 2

 Published in the
Published in the













 In September, the most read stories from Vascular Specialist included a change in leadership for the Midwestern Vascular Surgical Society; study results examining new treatment options for chronic limb-threatening ischemia (CLTI) patients; a call for more Spanish-speaking vascular surgeons, and several more.
In September, the most read stories from Vascular Specialist included a change in leadership for the Midwestern Vascular Surgical Society; study results examining new treatment options for chronic limb-threatening ischemia (CLTI) patients; a call for more Spanish-speaking vascular surgeons, and several more. 



 The Association of Program Directors in Vascular Surgery (APDVS) has released a new ebook designed to enhance
The Association of Program Directors in Vascular Surgery (APDVS) has released a new ebook designed to enhance 
 A foundational element for effective advocacy is understanding the profound impact that legislative decisions have on the practice of medicine and the health of our patients. The retirement announcements of several key members of
A foundational element for effective advocacy is understanding the profound impact that legislative decisions have on the practice of medicine and the health of our patients. The retirement announcements of several key members of 




















































 The
The 
 The
The 







 The European Society of Cardiology (
The European Society of Cardiology (


















 Vascular Therapies has today announced completion of enrollment in the
Vascular Therapies has today announced completion of enrollment in the 












 The Society for Vascular Surgery (
The Society for Vascular Surgery (
 “Life over limb”—that was one of the key messages to emerge from the
“Life over limb”—that was one of the key messages to emerge from the 
 The E. Stanley Crawford critical issues forum at
The E. Stanley Crawford critical issues forum at 
 Stakeholder organizations such as the Society for Vascular Surgery (
Stakeholder organizations such as the Society for Vascular Surgery (


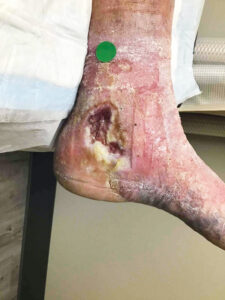
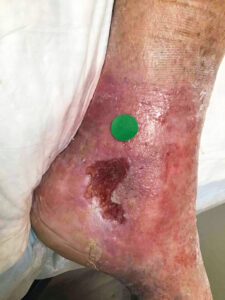
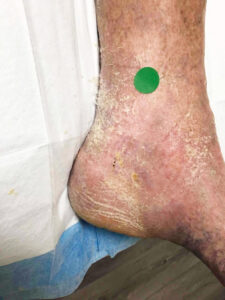




 Attendees of the
Attendees of the 
 The Society for Vascular Surgery (
The Society for Vascular Surgery ( Bhagwan Satiani, MD, explores the entrance of insurance companies into the physician practice acquisition market.
Bhagwan Satiani, MD, explores the entrance of insurance companies into the physician practice acquisition market.




 In June, the most read stories from Vascular Specialist included key reports from the 2024
In June, the most read stories from Vascular Specialist included key reports from the 2024 





 Those looking for a comprehensive review resource for vascular surgery are encouraged to check out the Society for Vascular Surgery’s (SVS) sixth edition of the Vascular Education and Self-Assessment Program (VESAP6).
Those looking for a comprehensive review resource for vascular surgery are encouraged to check out the Society for Vascular Surgery’s (SVS) sixth edition of the Vascular Education and Self-Assessment Program (VESAP6).


























 Five-year outcomes from the PRESERVE II study support the safety and effectiveness of the investigational Zenith iliac branch graft (ZBIS, Cook Medical) in combination with the iCast covered stent (Getinge) to preserve internal iliac artery perfusion during endovascular aneurysm repair (EVAR).
Five-year outcomes from the PRESERVE II study support the safety and effectiveness of the investigational Zenith iliac branch graft (ZBIS, Cook Medical) in combination with the iCast covered stent (Getinge) to preserve internal iliac artery perfusion during endovascular aneurysm repair (EVAR).























 The society for vascular nursing (SVN) is in the midst of its 42nd Annual Conference at McCormick Place, West. The conference kicked off Wednesday morning with the Presidential Welcome from Kristen Alix, MS, ANPBC, AGACNP, CVN, and was followed by the keynote address, “Disrupting DEI in a disruptive environment,” delivered by Katie Boston Leary, PhD, MBA, MHA, RN, NEA-BC.
The society for vascular nursing (SVN) is in the midst of its 42nd Annual Conference at McCormick Place, West. The conference kicked off Wednesday morning with the Presidential Welcome from Kristen Alix, MS, ANPBC, AGACNP, CVN, and was followed by the keynote address, “Disrupting DEI in a disruptive environment,” delivered by Katie Boston Leary, PhD, MBA, MHA, RN, NEA-BC.



















 The advent of
The advent of 
 The focus of yesterday’s American Pediatric Surgical Association (APSA)-SVS task force’s spring quarterly interest group meeting at
The focus of yesterday’s American Pediatric Surgical Association (APSA)-SVS task force’s spring quarterly interest group meeting at 










 This year’s James S. T. Yao Resident Research Award winner looks at novel therapeutic target for non-healing diabetic wounds. Kevin D. Mangum, MD, PhD, speaks to VS@VAM about his paper ahead of presenting the work at the 2024 Vascular Annual Meeting (VAM).
This year’s James S. T. Yao Resident Research Award winner looks at novel therapeutic target for non-healing diabetic wounds. Kevin D. Mangum, MD, PhD, speaks to VS@VAM about his paper ahead of presenting the work at the 2024 Vascular Annual Meeting (VAM).




 An education session set to take place in the afternoon of Wednesday, June 19 (3:15–4:45 p.m. in the
An education session set to take place in the afternoon of Wednesday, June 19 (3:15–4:45 p.m. in the 































 Attending a conference
Attending a conference 














 New data from the C-GUARDIANS pivotal investigational device exemption (IDE) trial support consideration of
New data from the C-GUARDIANS pivotal investigational device exemption (IDE) trial support consideration of 

 New multisociety clinical practice guidelines for the management of lower extremity
New multisociety clinical practice guidelines for the management of lower extremity 

 New clinical results highlight the need for inclusive approaches and comprehensive examinations of treatment options for
New clinical results highlight the need for inclusive approaches and comprehensive examinations of treatment options for 





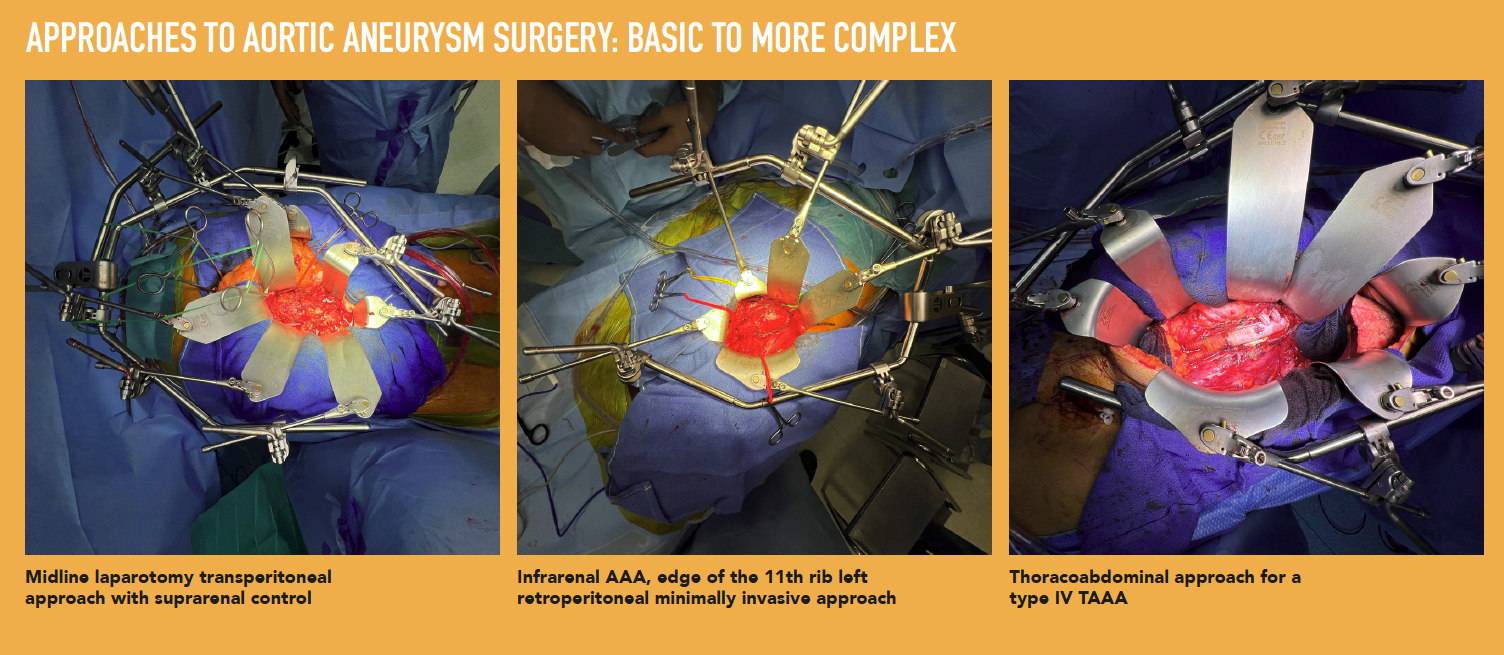









 The
The 







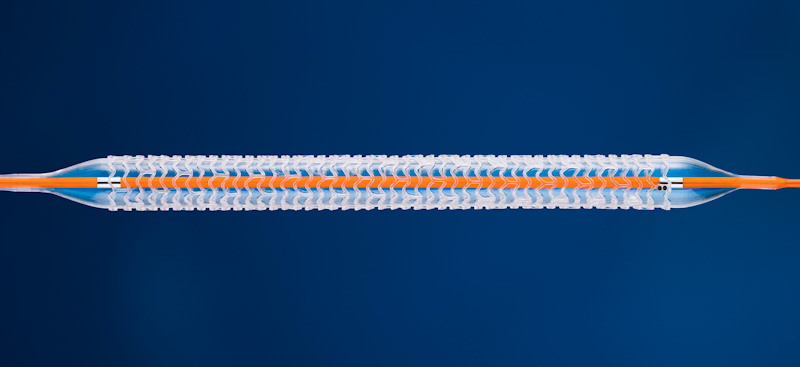
 Abbott has announced that the US Food and Drug Administration (FDA) has approved the Esprit BTK everolimus-eluting resorbable scaffold system (Esprit BTK system), a breakthrough innovation for people with chronic limb-threatening ischemia (CLTI) below-the-knee (BTK).
Abbott has announced that the US Food and Drug Administration (FDA) has approved the Esprit BTK everolimus-eluting resorbable scaffold system (Esprit BTK system), a breakthrough innovation for people with chronic limb-threatening ischemia (CLTI) below-the-knee (BTK).








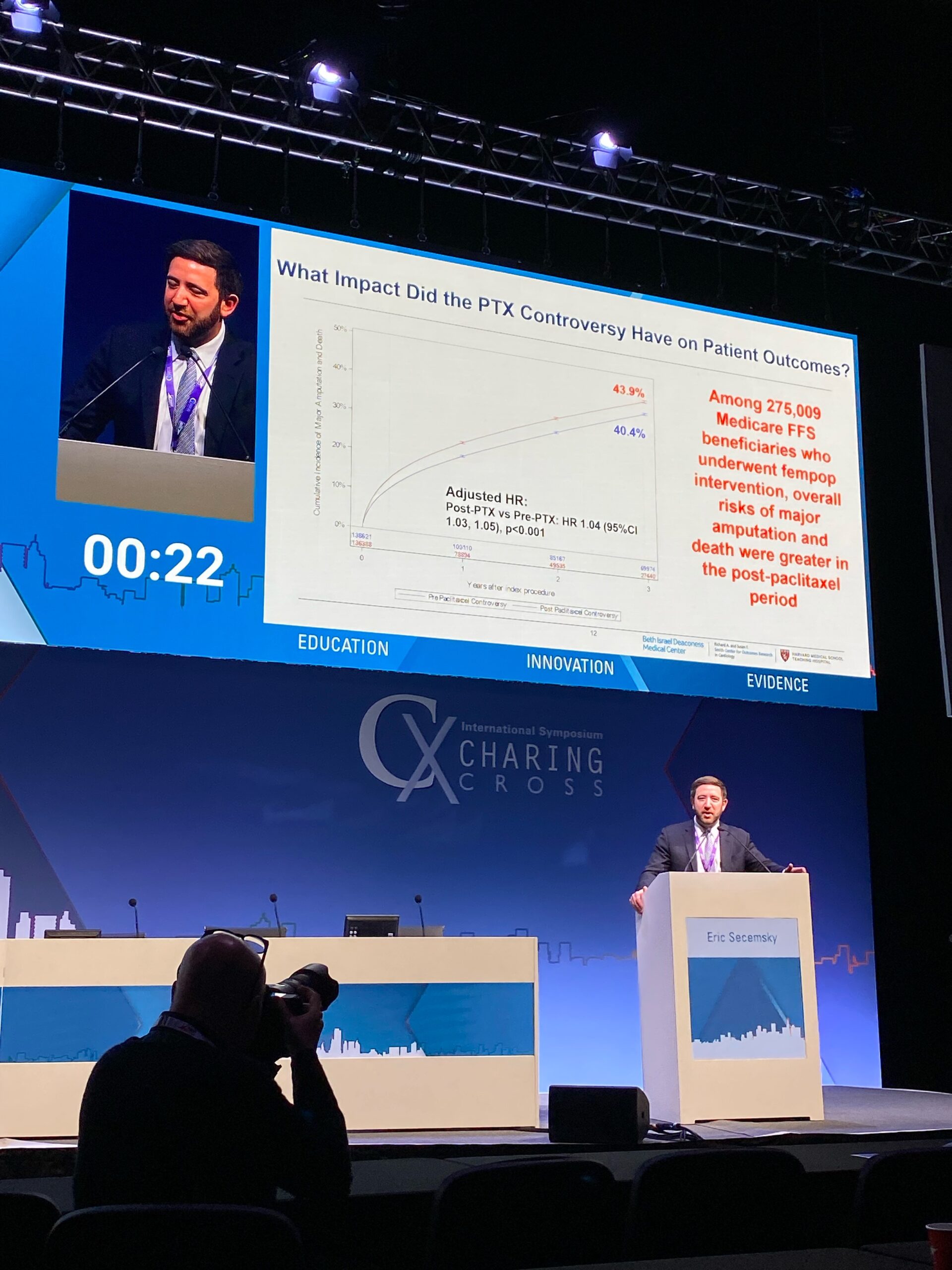



























 Johnson & Johnson is to acquire Shockwave Medical, it has been announced today.
Johnson & Johnson is to acquire Shockwave Medical, it has been announced today.










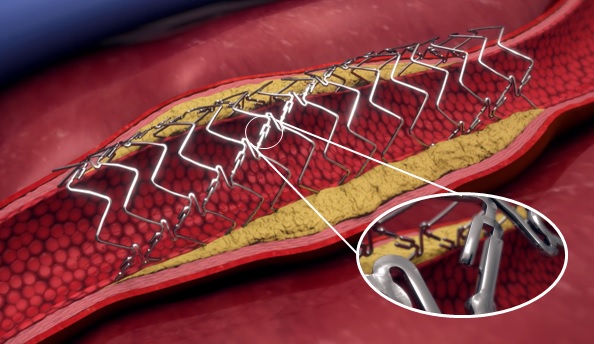











 Ruth Bush, MD, associate dean in the Office of Educational Affairs at John Sealy School of Medicine, the University of Texas Medical Branch (UTMB), in Galveston, Texas, took over as
Ruth Bush, MD, associate dean in the Office of Educational Affairs at John Sealy School of Medicine, the University of Texas Medical Branch (UTMB), in Galveston, Texas, took over as  Ticket information is available for the
Ticket information is available for the  The Society for Vascular Surgery (
The Society for Vascular Surgery ( Society for Vascular Surgery (
Society for Vascular Surgery (
 Filip Swirski, PhD, a leading figure in cardiovascular immunology research, will deliver this year’s Alexander W. Clowes Distinguished Lecture at the 2024 Vascular Research Initiatives Conference (
Filip Swirski, PhD, a leading figure in cardiovascular immunology research, will deliver this year’s Alexander W. Clowes Distinguished Lecture at the 2024 Vascular Research Initiatives Conference (
 Data from a new study that maps out the levels at which adverse postoperative events decrease following
Data from a new study that maps out the levels at which adverse postoperative events decrease following 
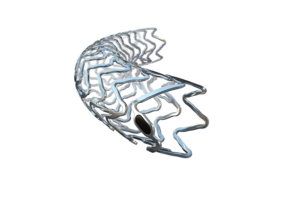



 In the U.S., the legislative process is complex. Among the most important in this process
In the U.S., the legislative process is complex. Among the most important in this process 









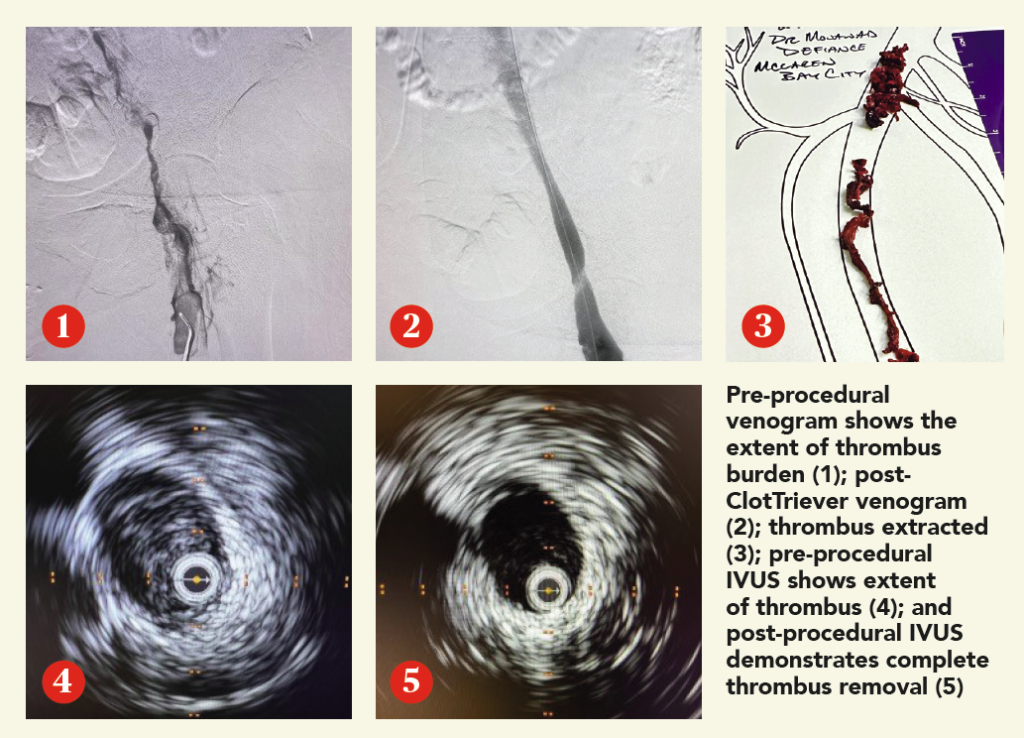 CASE REPORT
CASE REPORT








 In February, the most read stories from Vascular Specialist include a study of metformin and its potential as the first-ever medical treatment that can manage aneurysm disease; the SVS Sub-Section on Outpatient and Office Vascular Care (SOOVC) release the highly anticipated Office-Based Lab (OBL) Handbook; and a case series evaluation of aortoiliac endarterectomy, among several more.
In February, the most read stories from Vascular Specialist include a study of metformin and its potential as the first-ever medical treatment that can manage aneurysm disease; the SVS Sub-Section on Outpatient and Office Vascular Care (SOOVC) release the highly anticipated Office-Based Lab (OBL) Handbook; and a case series evaluation of aortoiliac endarterectomy, among several more. 

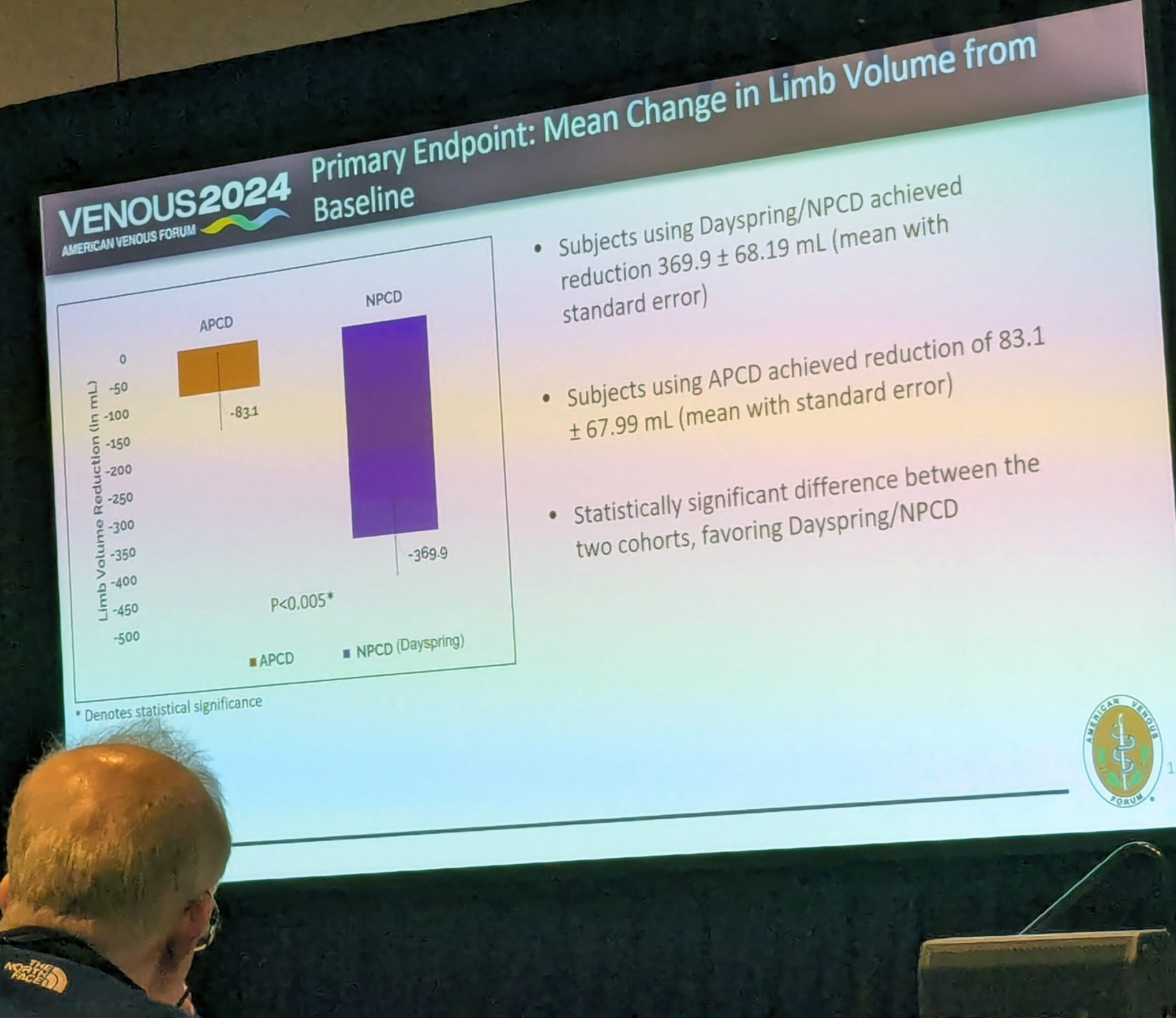






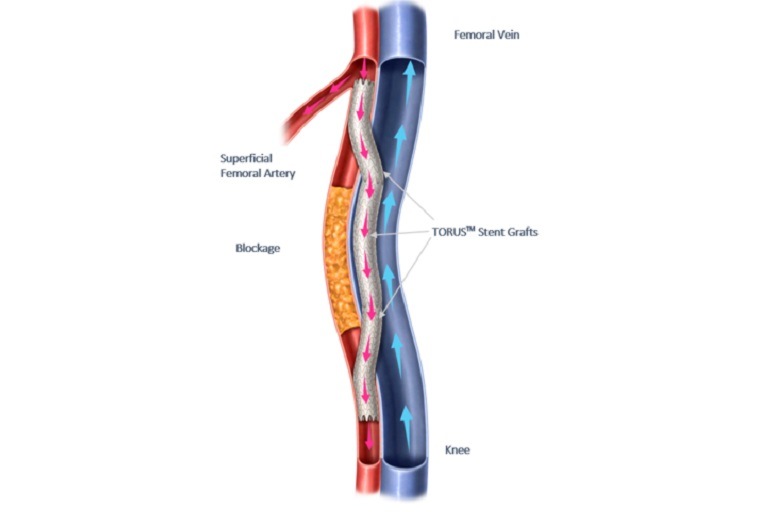
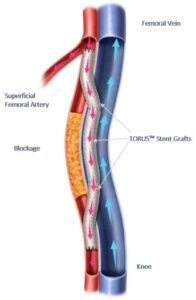





 In a retrospective review of over 600 patients who underwent iliac vein stent placement, the development of back pain was found to be unrelated to stent type, diameter, length or covered vein territory.
In a retrospective review of over 600 patients who underwent iliac vein stent placement, the development of back pain was found to be unrelated to stent type, diameter, length or covered vein territory.

 The Journal of Vascular Surgery (
The Journal of Vascular Surgery ( The Journal of Vascular Surgery Cases, Innovations and Techniques (
The Journal of Vascular Surgery Cases, Innovations and Techniques (
 In the fast-evolving landscape of medical education, podcasts have emerged as a powerful medium for disseminating knowledge and fostering community among professionals. Among these,
In the fast-evolving landscape of medical education, podcasts have emerged as a powerful medium for disseminating knowledge and fostering community among professionals. Among these, 
 So, what is budget neutrality and how does it affect physician payment? The Medicare Physician Fee Schedule (PFS) is a resource-based relative value scale (RVS) payment system, where relative value units (RVUs) are based on resource costs associated with physician work, practice expense and malpractice. The PFS has a budget neutrality requirement that has been in place since 1992. Budget neutrality is the federal mandate that requires an upward adjustment in expenditures in one area of the Medicare program to be offset by a downward adjustment in other areas. In the simplest terms, budget neutrality requires that the Centers for Medicare & Medicaid Services (
So, what is budget neutrality and how does it affect physician payment? The Medicare Physician Fee Schedule (PFS) is a resource-based relative value scale (RVS) payment system, where relative value units (RVUs) are based on resource costs associated with physician work, practice expense and malpractice. The PFS has a budget neutrality requirement that has been in place since 1992. Budget neutrality is the federal mandate that requires an upward adjustment in expenditures in one area of the Medicare program to be offset by a downward adjustment in other areas. In the simplest terms, budget neutrality requires that the Centers for Medicare & Medicaid Services (






 A randomized controlled trial (
A randomized controlled trial (
 In response to recent articles in the lay press suggesting vascular surgeons were performing unnecessary procedures, Vascular Specialist medical editor, Malachi Sheahan, has asked me to write an editorial based on a talk I presented at the
In response to recent articles in the lay press suggesting vascular surgeons were performing unnecessary procedures, Vascular Specialist medical editor, Malachi Sheahan, has asked me to write an editorial based on a talk I presented at the 

 Sensome has announced enrollment of the first patients into a feasibility clinical study using the Clotild smart guidewire in
Sensome has announced enrollment of the first patients into a feasibility clinical study using the Clotild smart guidewire in 




 An upcoming
An upcoming 




 The use of a relatively novel scoring system, referred to as Symptomatic carotid atheroma inflammation lumen-stenosis (SCAIL), has produced favourable predictive qualities versus the Oxford carotid stenosis tool (OCST) and Essen stroke risk score (ESRS). According to researchers, the SCAIL score led to superior recurrent stroke predictions after minor stroke/transient ischaemic attack (TIA) and symptomatic
The use of a relatively novel scoring system, referred to as Symptomatic carotid atheroma inflammation lumen-stenosis (SCAIL), has produced favourable predictive qualities versus the Oxford carotid stenosis tool (OCST) and Essen stroke risk score (ESRS). According to researchers, the SCAIL score led to superior recurrent stroke predictions after minor stroke/transient ischaemic attack (TIA) and symptomatic 
 Following a review, the United Kingdom’s Medicines and Healthcare Products Regulatory Agency (MHRA) has updated guidance on the use of
Following a review, the United Kingdom’s Medicines and Healthcare Products Regulatory Agency (MHRA) has updated guidance on the use of 


 The
The  The
The 







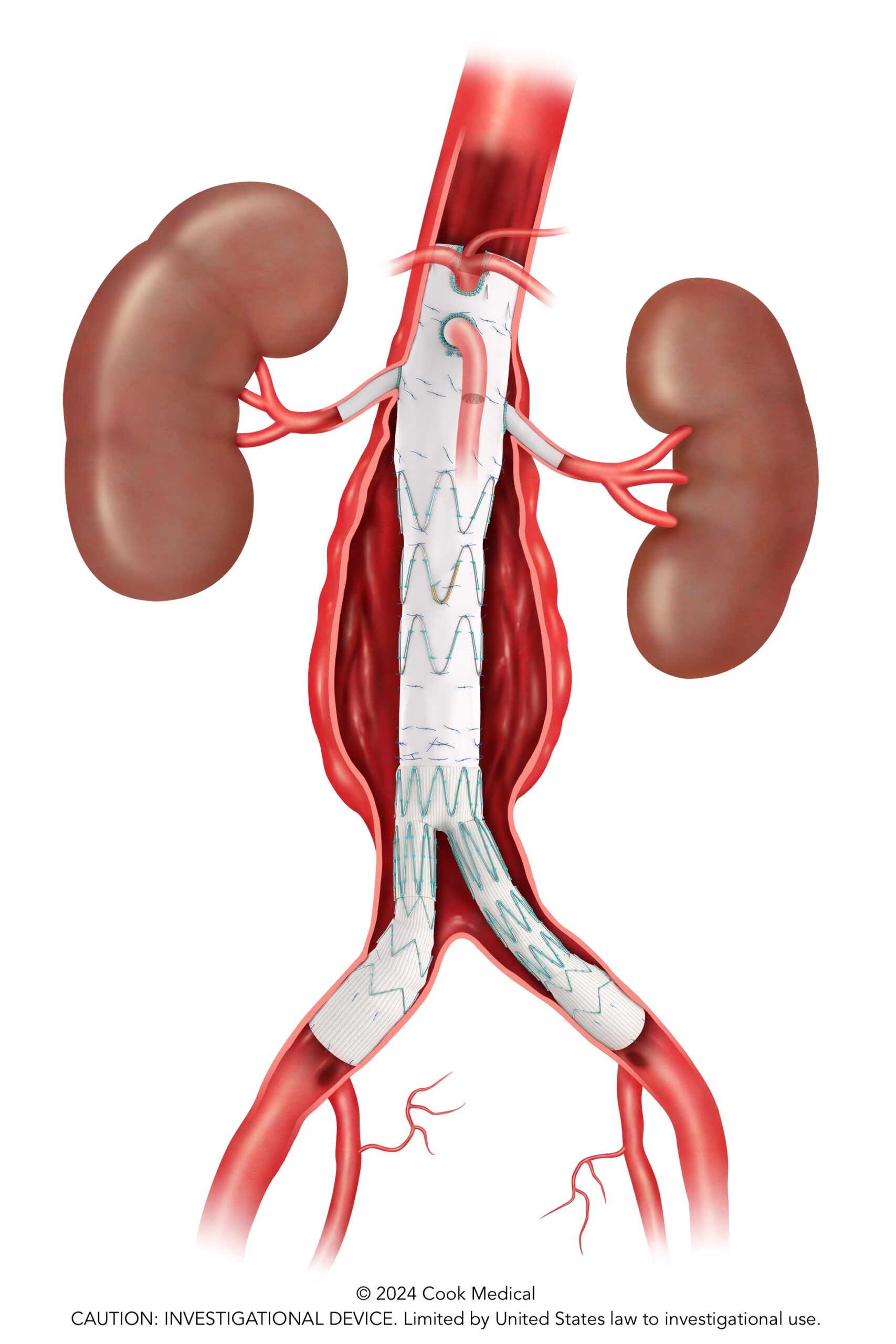
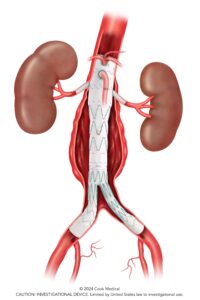








 T
T



 A vascular surgery team at Audie L. Murphy VA Medical Center in San Antonio, Texas, uncovers “staggeringly low” numbers of correctly coded billing for three commonly performed vascular procedures, raising concerns over cases with more complex coding.
A vascular surgery team at Audie L. Murphy VA Medical Center in San Antonio, Texas, uncovers “staggeringly low” numbers of correctly coded billing for three commonly performed vascular procedures, raising concerns over cases with more complex coding.
 The final publication of midterm results from the observational CARAS study suggest that cerebral ischaemic event (CIE) incidence in
The final publication of midterm results from the observational CARAS study suggest that cerebral ischaemic event (CIE) incidence in 









 Endovascular Engineering (E2) has announced US Food and Drug Administration (FDA) investigational device exemption (IDE) approval for its ENGULF US pivotal trial.
Endovascular Engineering (E2) has announced US Food and Drug Administration (FDA) investigational device exemption (IDE) approval for its ENGULF US pivotal trial.


 Which stories captured the attention of the vascular community over the course of the past year? Read our summary of the trending articles from across Vascular Specialist in 2023. Let us know your highlights by leaving a reply at the foot of the page with your comments.
Which stories captured the attention of the vascular community over the course of the past year? Read our summary of the trending articles from across Vascular Specialist in 2023. Let us know your highlights by leaving a reply at the foot of the page with your comments.





 “We’re going to see more and more amputations if we don’t figure out the right post procedure thromboprophylaxis regime ASAP,” Anahita Dua, MD, warned during a lecture on innovative approaches to preventing
“We’re going to see more and more amputations if we don’t figure out the right post procedure thromboprophylaxis regime ASAP,” Anahita Dua, MD, warned during a lecture on innovative approaches to preventing 









 T
T












 Researchers may now submit abstracts for the SVS Vascular Research Initiatives Conference (
Researchers may now submit abstracts for the SVS Vascular Research Initiatives Conference (














 This summer the center for medicare and Medicaid Services (
This summer the center for medicare and Medicaid Services (


 I enjoy listening to podcasts. In addition to my usual rotation through Audible Bleeding and Behind the Knife, I’ve found insight in listening to Hidden Brain. A recent episode centered on the concept of gratitude, where it was highlighted how quick we are to dismiss the good things that happen to us and perseverate on the negative, inconvenient or unsavory parts of our day—both for ourselves and those around us.
I enjoy listening to podcasts. In addition to my usual rotation through Audible Bleeding and Behind the Knife, I’ve found insight in listening to Hidden Brain. A recent episode centered on the concept of gratitude, where it was highlighted how quick we are to dismiss the good things that happen to us and perseverate on the negative, inconvenient or unsavory parts of our day—both for ourselves and those around us. 
 Periodically, when under pressure at work, and sometimes at home, I would think about what I could do to free up more time for myself and the family. How I could prioritize or drop tasks that were unimportant. There were and are many books and articles advising us how to prioritize assignments. Over time, most of us have devised an imperfect system to deal with non-urgent tasks. A high-level approach to separate the chaff from the wheat may start with things such as your own goals, organizational objectives followed by a variety of individual objectives. However, the day-to-day pesky problems added to our calendar continue to test our time-management skills.
Periodically, when under pressure at work, and sometimes at home, I would think about what I could do to free up more time for myself and the family. How I could prioritize or drop tasks that were unimportant. There were and are many books and articles advising us how to prioritize assignments. Over time, most of us have devised an imperfect system to deal with non-urgent tasks. A high-level approach to separate the chaff from the wheat may start with things such as your own goals, organizational objectives followed by a variety of individual objectives. However, the day-to-day pesky problems added to our calendar continue to test our time-management skills.





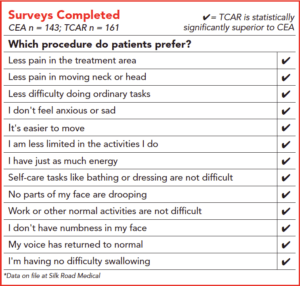


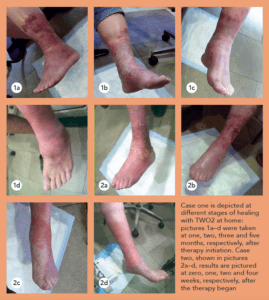 Case no. 1
Case no. 1



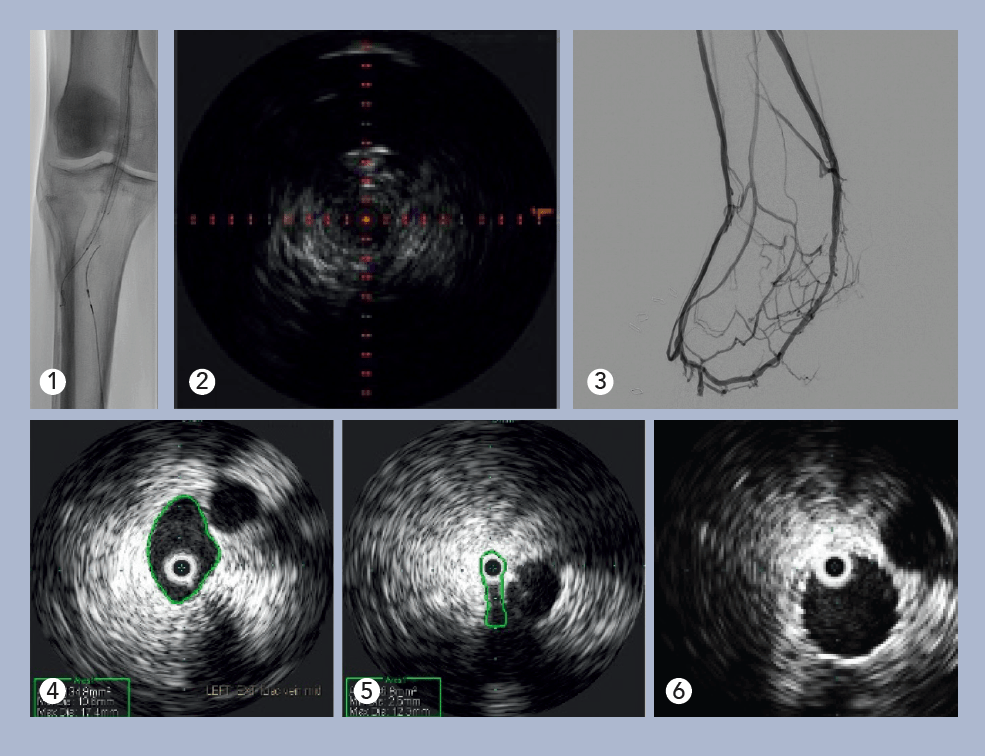
 Here, we present a case of a 65-year-old Caucasian female with a three-year history of left leg swelling, heaviness and achiness, which improved with elevation.
Here, we present a case of a 65-year-old Caucasian female with a three-year history of left leg swelling, heaviness and achiness, which improved with elevation.
 One-year results from the PROMISE II US pivotal trial demonstrate the durability of outcomes of transcatheter arterialisation of the deep veins (TADV) using the
One-year results from the PROMISE II US pivotal trial demonstrate the durability of outcomes of transcatheter arterialisation of the deep veins (TADV) using the 














 The SVS, in collaboration with the
The SVS, in collaboration with the 
 Data can be granular and clarifying. Conversely, data without appropriate context can lack nuance—like a paint-by-numbers portrait using only one crayon.
Data can be granular and clarifying. Conversely, data without appropriate context can lack nuance—like a paint-by-numbers portrait using only one crayon. With the congressional “busy season” upon us, the SVS is gearing up for big tasks ahead on the legislative and regulatory fronts. Having stepped into the role of chair of the SVS Advocacy Council this past June, I have to start by highlighting the generosity of our members and the service of surgeon-volunteers who have provided an incredibly strong foundation to build on for the work that lies ahead.
With the congressional “busy season” upon us, the SVS is gearing up for big tasks ahead on the legislative and regulatory fronts. Having stepped into the role of chair of the SVS Advocacy Council this past June, I have to start by highlighting the generosity of our members and the service of surgeon-volunteers who have provided an incredibly strong foundation to build on for the work that lies ahead.

 Applications open this month for the SVS Excellence in Community Practice Award and the Sub-Section on Outpatient & Office Vascular Care (SOOVC) Research Seed Grant and Presentation Award.
Applications open this month for the SVS Excellence in Community Practice Award and the Sub-Section on Outpatient & Office Vascular Care (SOOVC) Research Seed Grant and Presentation Award. 






 Julie A. Freischlag, MD, used her turn as honorary guest lecturer at the 2023 Midwestern Vascular Surgical Society (
Julie A. Freischlag, MD, used her turn as honorary guest lecturer at the 2023 Midwestern Vascular Surgical Society (













 T
T











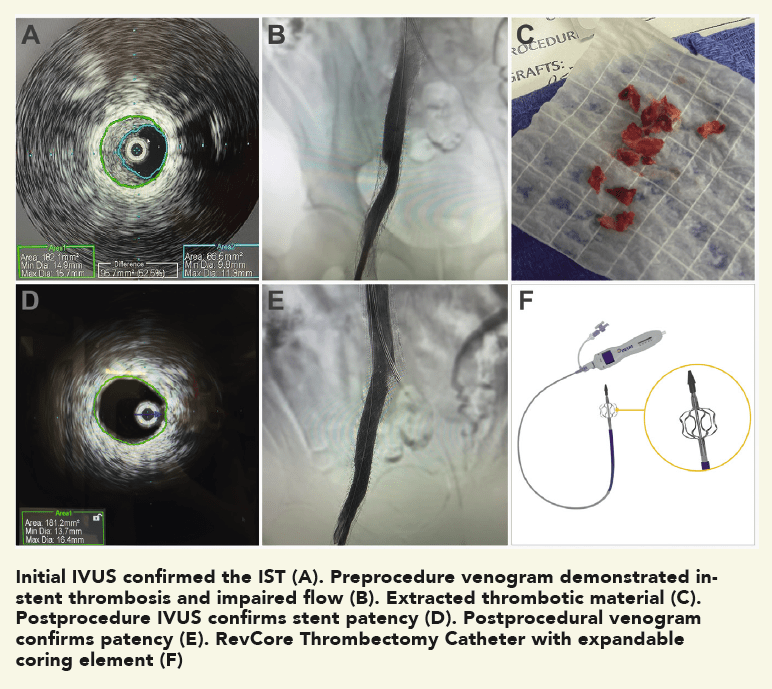 Procedural overview
Procedural overview











 The
The

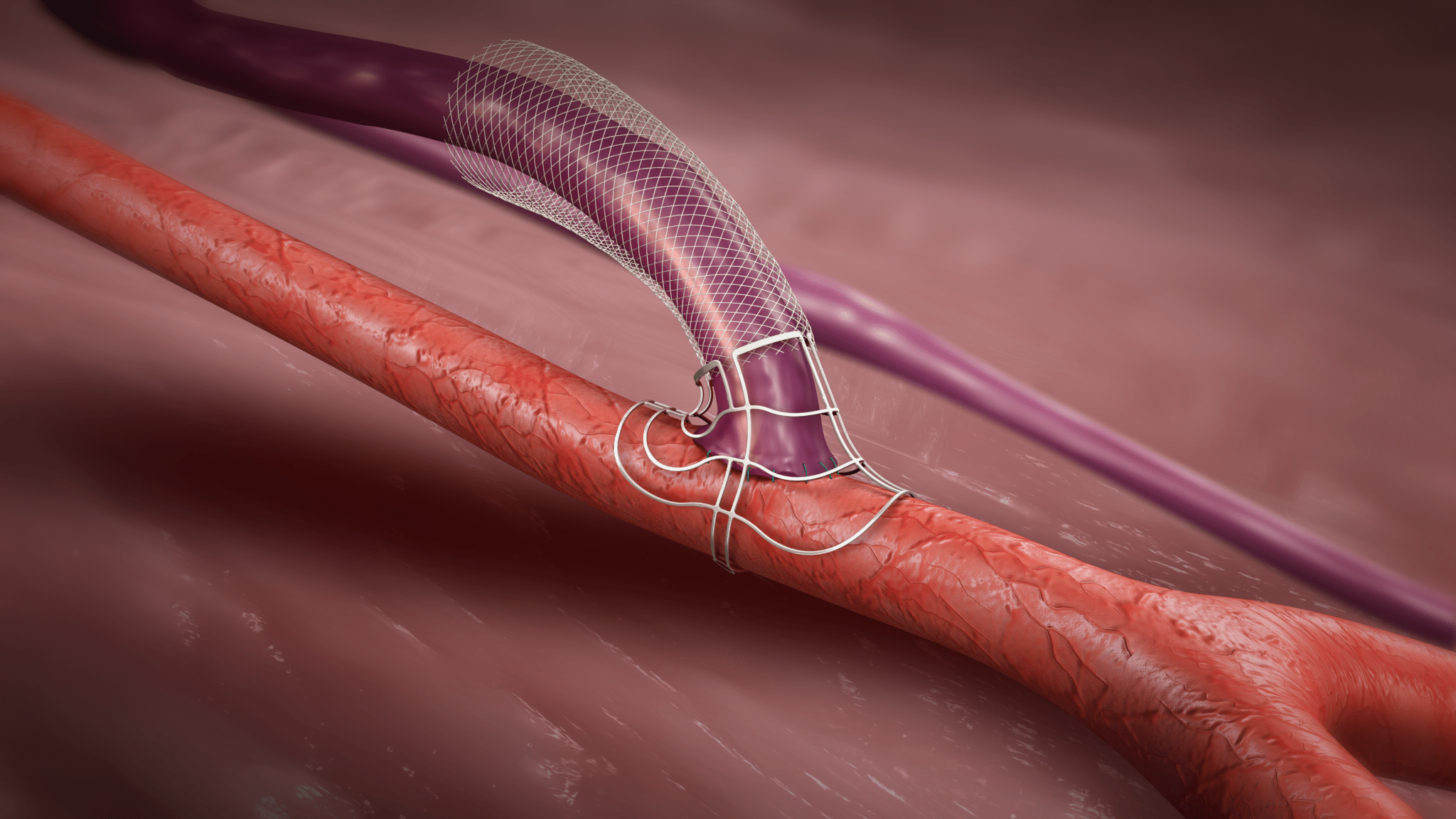






 The 2024 Society for for Vascular Surgery (SVS)
The 2024 Society for for Vascular Surgery (SVS) 




















 The month of September begins Friday and with it, the SVS Foundation’s
The month of September begins Friday and with it, the SVS Foundation’s 








 In a recently published study, dedicated venous stents performed well through
In a recently published study, dedicated venous stents performed well through 












 Merit Medical has announced that it has completed enrollment in its WRAPSODY Arteriovenous Access Efficacy (WAVE) pivotal study. Merit’s WAVE study is a prospective, randomized, controlled, multicenter study comparing the Merit WRAPSODY cell-impermeable endoprosthesis (CIE) to percutaneous transluminal angioplasty (PTA) for treatment of stenosis/occlusion in the venous outflow circuit in patients undergoing hemodialysis.
Merit Medical has announced that it has completed enrollment in its WRAPSODY Arteriovenous Access Efficacy (WAVE) pivotal study. Merit’s WAVE study is a prospective, randomized, controlled, multicenter study comparing the Merit WRAPSODY cell-impermeable endoprosthesis (CIE) to percutaneous transluminal angioplasty (PTA) for treatment of stenosis/occlusion in the venous outflow circuit in patients undergoing hemodialysis. 






 The Vascular and Endovascular Devices Team (VEDT) in the
The Vascular and Endovascular Devices Team (VEDT) in the  In the previous month, Vascular Specialist delivered a range of leading commentary from the Society for Vascular Surgery (SVS) in response to the New York Times investigation on inappropriateness in vascular care; the anticipated Food and Drug Administration (FDA) update on excess mortality risk for paclitaxel-coated devices; a review of protocols addressing hypersensitivity reactions after cyanoacrylate varicose vein treatment; and rumination from the E. Stanley Crawford Critical Issues Forum on the pipeline of sources feeding the vascular surgery ranks.
In the previous month, Vascular Specialist delivered a range of leading commentary from the Society for Vascular Surgery (SVS) in response to the New York Times investigation on inappropriateness in vascular care; the anticipated Food and Drug Administration (FDA) update on excess mortality risk for paclitaxel-coated devices; a review of protocols addressing hypersensitivity reactions after cyanoacrylate varicose vein treatment; and rumination from the E. Stanley Crawford Critical Issues Forum on the pipeline of sources feeding the vascular surgery ranks.


 The number of
The number of 







 Nectero Medical today announced that the US Food and Drug Administration (FDA) has granted investigational new drug (IND) clearance for the company to initiate a prospective, multicentre, randomised clinical trial (the stAAAble study) to evaluate the safety and efficacy of the Nectero endovascular aneurysm stabilisation treatment (EAST) system in patients with infrarenal
Nectero Medical today announced that the US Food and Drug Administration (FDA) has granted investigational new drug (IND) clearance for the company to initiate a prospective, multicentre, randomised clinical trial (the stAAAble study) to evaluate the safety and efficacy of the Nectero endovascular aneurysm stabilisation treatment (EAST) system in patients with infrarenal 






 Uncertainty underlying the magnitude of risk posed by long distance air travel in
Uncertainty underlying the magnitude of risk posed by long distance air travel in 



 Last month, Vascular Specialist garnered readers’ attention with an array of commentary on the BEST-CLI and BASIL-2 trials; the medical editor’s interview with vascular surgery’s ‘living legend’ Frank J. Veith, MD; the news of the death of Past SVS President Kenneth Wayne Johnston; and a report of a Vascular Annual Meeting (VAM) breakfast session on the role of vascular surgeons in the pulmonary embolism (PE) treatment paradigm.
Last month, Vascular Specialist garnered readers’ attention with an array of commentary on the BEST-CLI and BASIL-2 trials; the medical editor’s interview with vascular surgery’s ‘living legend’ Frank J. Veith, MD; the news of the death of Past SVS President Kenneth Wayne Johnston; and a report of a Vascular Annual Meeting (VAM) breakfast session on the role of vascular surgeons in the pulmonary embolism (PE) treatment paradigm.










 A new analysis of
A new analysis of 
 A recent study concludes that non-thrombotic iliac vein lesion (NIVL) patients have better primary patency after venous stenting than patients with
A recent study concludes that non-thrombotic iliac vein lesion (NIVL) patients have better primary patency after venous stenting than patients with 





 A recent survey has indicated that physical pain is “prevalent” among vascular surgery trainees and represents a risk factor for
A recent survey has indicated that physical pain is “prevalent” among vascular surgery trainees and represents a risk factor for 
























 A night of grandeur, reminiscent of the parties hosted by the fictional character Jay Gatsby, awaits attendees on tonight (Friday) at the “Great Gatsby Gala,” benefiting the SVS Foundation.
A night of grandeur, reminiscent of the parties hosted by the fictional character Jay Gatsby, awaits attendees on tonight (Friday) at the “Great Gatsby Gala,” benefiting the SVS Foundation. 
 The annual business meeting (for members only).
The annual business meeting (for members only).