 RapidAI today announced the US Food and Drug Administration (FDA) clearance of Aortic Management, part of Rapid Aortic, which the company describes as “a comprehensive deep clinical AI [artificial intelligence] solution designed to transform the acute assessment and longitudinal management of aortic disease”.
RapidAI today announced the US Food and Drug Administration (FDA) clearance of Aortic Management, part of Rapid Aortic, which the company describes as “a comprehensive deep clinical AI [artificial intelligence] solution designed to transform the acute assessment and longitudinal management of aortic disease”.
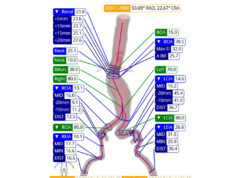
RapidAI shares that its Rapid Aortic product not only provides deep clinical information powered by AI to help clinicians “quickly and accurately” identify more aortic aneurysms, dissections, and other anomalies, but also automatically generates critical measurements, produces automated 3D reconstructions of the entire aorta, and tracks anatomical changes over time.
“Rapid Aortic represents a significant advancement for aortic patient management,” said vascular surgeon Trissa Babrowski (University of Chicago, Chicago, USA). “With instant access to every textbook landmark and zonal maximum measurement, advanced visualisations, and robust longitudinal tracking for surveillance, Rapid Aortic empowers physicians to efficiently identify, confidently assess, and diligently monitor every aortic patient within their system.”
RapidAI specifies that Rapid Aortic is fully integrated into the Rapid Edge Cloud, which the company states is the first cloud-based IT platform with on-prem capabilities that ensures continuous service during any disruptions. Additionally, RapidAI notes that all modules are integrated into Rapid Navigator Pro, the company’s next-generation radiology solution, as well as the Rapid mobile and web applications.